Sleep as a Potential Biomarker of Tau and β-Amyloid Burden in the Human Brain
- PMID: 31209175
- PMCID: PMC6687908
- DOI: 10.1523/JNEUROSCI.0503-19.2019
Sleep as a Potential Biomarker of Tau and β-Amyloid Burden in the Human Brain
Abstract
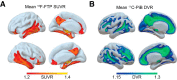
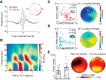
Recent proposals suggest that sleep may be a factor associated with accumulation of two core pathological features of Alzheimer's disease (AD): tau and β-amyloid (Aβ). Here we combined PET measures of Aβ and tau, electroencephalogram sleep recordings, and retrospective sleep evaluations to investigate the potential utility of sleep measures in predicting in vivo AD pathology in male and female older adults. Regression analyses revealed that the severity of impaired slow oscillation-sleep spindle coupling predicted greater medial temporal lobe tau burden. Aβ burden was not associated with coupling impairment but instead predicted the diminished amplitude of <1 Hz slow-wave-activity, results that were statistically dissociable from each other. Additionally, comparisons of AD pathology and retrospective, self-reported changes in sleep duration demonstrated that changes in sleep across the lifespan can predict late-life Aβ and tau burden. Thus, quantitative and qualitative features of human sleep represent potential noninvasive, cost-effective, and scalable biomarkers (current and future forecasting) of AD pathology, and carry both therapeutic and public health implications.SIGNIFICANCE STATEMENT Several studies have linked sleep disruption to the progression of Alzheimer's disease (AD). Tau and β-amyloid (Aβ), the primary pathological features of AD, are associated with both objective and subjective changes in sleep. However, it remains unknown whether late life tau and Aβ burden are associated with distinct impairments in sleep physiology or changes in sleep across the lifespan. Using polysomnography, retrospective questionnaires, and tau- and Aβ-specific PET, the present study reveals human sleep signatures that dissociably predict levels of brain tau and Aβ in older adults. These results suggest that a night of polysomnography may aid in evaluating tau and Aβ burden, and that treating sleep deficiencies within decade-specific time windows may serve in delaying AD progression.
Keywords: Alzheimer's disease; PET; aging; beta-amyloid; sleep; tau.
Copyright © 2019 the authors.
Figures


Similar articles
-
Subthreshold Amyloid Predicts Tau Deposition in Aging.J Neurosci. 2018 May 9;38(19):4482-4489. doi: 10.1523/JNEUROSCI.0485-18.2018. Epub 2018 Apr 23. J Neurosci. 2018. PMID: 29686045 Free PMC article.
-
Accuracy of Tau Positron Emission Tomography as a Prognostic Marker in Preclinical and Prodromal Alzheimer Disease: A Head-to-Head Comparison Against Amyloid Positron Emission Tomography and Magnetic Resonance Imaging.JAMA Neurol. 2021 Aug 1;78(8):961-971. doi: 10.1001/jamaneurol.2021.1858. JAMA Neurol. 2021. PMID: 34180956 Free PMC article.
-
Region-Specific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden.JAMA Neurol. 2017 Dec 1;74(12):1455-1463. doi: 10.1001/jamaneurol.2017.2216. JAMA Neurol. 2017. PMID: 28973551 Free PMC article.
-
Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.Alzheimers Dement. 2019 Jan;15(1):106-152. doi: 10.1016/j.jalz.2018.08.005. Epub 2018 Oct 13. Alzheimers Dement. 2019. PMID: 30321505 Review.
-
Correlation of Alzheimer Disease Neuropathologic Staging with Amyloid and Tau Scintigraphic Imaging Biomarkers.J Nucl Med. 2020 Oct;61(10):1413-1418. doi: 10.2967/jnumed.119.230458. Epub 2020 Aug 6. J Nucl Med. 2020. PMID: 32764121 Review.
Cited by
-
Sleep-Based Interventions in Alzheimer's Disease: Promising Approaches from Prevention to Treatment along the Disease Trajectory.Pharmaceuticals (Basel). 2021 Apr 19;14(4):383. doi: 10.3390/ph14040383. Pharmaceuticals (Basel). 2021. PMID: 33921870 Free PMC article. Review.
-
Local Sleep and Alzheimer's Disease Pathophysiology.Front Neurosci. 2020 Sep 23;14:525970. doi: 10.3389/fnins.2020.525970. eCollection 2020. Front Neurosci. 2020. PMID: 33071726 Free PMC article. Review.
-
Obstructive Sleep Apnea and Its Treatment in Aging: Effects on Alzheimer's disease Biomarkers, Cognition, Brain Structure and Neurophysiology.Neurobiol Dis. 2020 Nov;145:105054. doi: 10.1016/j.nbd.2020.105054. Epub 2020 Aug 27. Neurobiol Dis. 2020. PMID: 32860945 Free PMC article. Review.
-
New perspectives on the basal forebrain cholinergic system in Alzheimer's disease.Neurosci Biobehav Rev. 2023 Jul;150:105192. doi: 10.1016/j.neubiorev.2023.105192. Epub 2023 Apr 20. Neurosci Biobehav Rev. 2023. PMID: 37086935 Free PMC article. Review.
-
Co-ordination of brain and heart oscillations during non-rapid eye movement sleep.J Sleep Res. 2022 Apr;31(2):e13466. doi: 10.1111/jsr.13466. Epub 2021 Aug 31. J Sleep Res. 2022. PMID: 34467582 Free PMC article.
References
-
- Ahnaou A, Moechars D, Raeymaekers L, Biermans R, Manyakov NV, Bottelbergs A, Wintmolders C, Van Kolen K, Van De Casteele T, Kemp JA, Drinkenburg WH (2017) Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer's disease pathology. Sci Rep 7:14189. 10.1038/s41598-017-13839-6 - DOI - PMC - PubMed
-
- Berens P. (2009) CircStat: a MATLAB toolbox for circular statistics. J Stat Softw 31:1–21.
