Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis
- PMID: 31209199
- PMCID: PMC6579767
- DOI: 10.1038/s41419-019-1678-y
Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis
Abstract
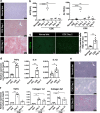
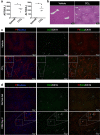
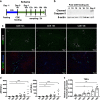
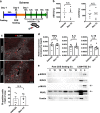
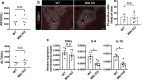
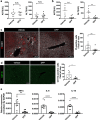
Nonalcoholic steatohepatitis (NASH) is a metabolic liver disease that progresses from simple steatosis to the disease state of inflammation and fibrosis. Previous studies suggest that apoptosis and necroptosis may contribute to the pathogenesis of NASH, based on several murine models. However, the mechanisms underlying the transition of simple steatosis to steatohepatitis remain unclear, because it is difficult to identify when and where such cell deaths begin to occur in the pathophysiological process of NASH. In the present study, our aim is to investigate which type of cell death plays a role as the trigger for initiating inflammation in fatty liver. By establishing a simple method of discriminating between apoptosis and necrosis in the liver, we found that necrosis occurred prior to apoptosis at the onset of steatohepatitis in the choline-deficient, ethionine-supplemented (CDE) diet model. To further investigate what type of necrosis is involved in the initial necrotic cell death, we examined the effect of necroptosis and ferroptosis inhibition by administering inhibitors to wild-type mice in the CDE diet model. In addition, necroptosis was evaluated using mixed lineage kinase domain-like protein (MLKL) knockout mice, which is lacking in a terminal executor of necroptosis. Consequently, necroptosis inhibition failed to block the onset of necrotic cell death, while ferroptosis inhibition protected hepatocytes from necrotic death almost completely, and suppressed the subsequent infiltration of immune cells and inflammatory reaction. Furthermore, the amount of oxidized phosphatidylethanolamine, which is involved in ferroptosis pathway, was increased in the liver sample of the CDE diet-fed mice. These findings suggest that hepatic ferroptosis plays an important role as the trigger for initiating inflammation in steatohepatitis and may be a therapeutic target for preventing the onset of steatohepatitis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
In Vivo Analysis of Necrosis and Ferroptosis in Nonalcoholic Steatohepatitis (NASH).Methods Mol Biol. 2022;2455:267-278. doi: 10.1007/978-1-0716-2128-8_21. Methods Mol Biol. 2022. PMID: 35213001
-
Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis.Clin Sci (Lond). 2015 Oct 1;129(8):721-39. doi: 10.1042/CS20140732. Epub 2015 Jun 15. Clin Sci (Lond). 2015. PMID: 26201023
-
Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease.J Hepatol. 2020 Apr;72(4):627-635. doi: 10.1016/j.jhep.2019.11.008. Epub 2019 Nov 21. J Hepatol. 2020. PMID: 31760070
-
[Cell death mechanisms in non-alcoholic steatohepatitis].Biol Aujourdhui. 2020;214(1-2):1-13. doi: 10.1051/jbio/2020002. Epub 2020 Aug 10. Biol Aujourdhui. 2020. PMID: 32773025 Review. French.
-
Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease.Adv Exp Med Biol. 2018;1061:19-44. doi: 10.1007/978-981-10-8684-7_3. Adv Exp Med Biol. 2018. PMID: 29956204 Review.
Cited by
-
Focus on the Role of Inflammation as a Bridge between Ferroptosis and Atrial Fibrillation: A Narrative Review and Novel Perspective.Rev Cardiovasc Med. 2024 Mar 25;25(4):110. doi: 10.31083/j.rcm2504110. eCollection 2024 Apr. Rev Cardiovasc Med. 2024. PMID: 39076556 Free PMC article. Review.
-
Efferocytosis in liver disease.JHEP Rep. 2023 Nov 16;6(1):100960. doi: 10.1016/j.jhepr.2023.100960. eCollection 2024 Jan. JHEP Rep. 2023. PMID: 38234410 Free PMC article. Review.
-
CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis.Sci Transl Med. 2022 Nov 23;14(672):eabp8309. doi: 10.1126/scitranslmed.abp8309. Epub 2022 Nov 23. Sci Transl Med. 2022. PMID: 36417485 Free PMC article.
-
The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice.Nat Commun. 2023 Jan 23;14(1):167. doi: 10.1038/s41467-023-35804-w. Nat Commun. 2023. PMID: 36690638 Free PMC article.
-
The Critical Role of Ferroptosis in Hepatocellular Carcinoma.Front Cell Dev Biol. 2022 Jun 21;10:882571. doi: 10.3389/fcell.2022.882571. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35800895 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

