Design and application of α-ketothioesters as 1,2-dicarbonyl-forming reagents
- PMID: 31209228
- PMCID: PMC6572800
- DOI: 10.1038/s41467-019-10651-w
Design and application of α-ketothioesters as 1,2-dicarbonyl-forming reagents
Abstract
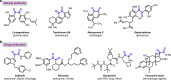
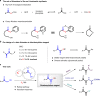
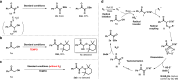
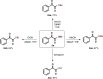
The 1,2-dicarbonyl motif is vital to biomolecules, especially natural products and pharmaceuticals. Conventionally, 1,2-dicarbonyl compounds are prepared via an α-keto acyl chloride. Based on the methods used in nature, a transition-metal-free approach for the synthesis of an α-ketothioester reagent via the combination of an α-hydroxyl ketone, elemental sulfur and a benzyl halide is reported. Mechanistic studies demonstrate that the trisulfur radical anion and the α-carbon radical of the α-hydroxy ketone are involved in this transformation. The dicarbonylation of a broad range of amines and amino acids, and importantly, cross couplings with aryl borates to construct dicarbonyl-carbon bonds are realized under mild conditions by employing this stable and convenient α-ketothioester as a 1,2-dicarbonyl reagent. The dicarbonyl-containing drug indibulin and the natural product polyandrocarpamide C, which possess multiple heteroatoms and active hydrogen functional groups, can be efficiently prepared using the designed 1,2-dicarbonyl reagent.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Direct organocatalytic asymmetric alpha-sulfenylation of activated C-H bonds in lactones, lactams, and beta-dicarbonyl compounds.Chemistry. 2005 Sep 19;11(19):5689-94. doi: 10.1002/chem.200500512. Chemistry. 2005. PMID: 16035005
-
Sc(OTf)3-catalyzed three-component cyclization of arylamines, β,γ-unsaturated α-ketoesters, and 1,3-dicarbonyl compounds for the synthesis of highly substituted 1,4-dihydropyridines and tetrahydropyridines.J Org Chem. 2013 Jun 7;78(11):5751-5. doi: 10.1021/jo400578m. Epub 2013 May 16. J Org Chem. 2013. PMID: 23614840
-
A C(sp3)-C(sp3) bond forming reductive condensation of α-keto esters and enolizable carbon pronucleophiles.Org Lett. 2013 Jun 21;15(12):3090-3. doi: 10.1021/ol401276b. Epub 2013 Jun 5. Org Lett. 2013. PMID: 23738614
-
Construction of contiguous tetrasubstituted chiral carbon stereocenters via direct catalytic asymmetric aldol and Mannich-type reactions.Chem Rec. 2011 Sep;11(5):260-8. doi: 10.1002/tcr.201100020. Epub 2011 Sep 6. Chem Rec. 2011. PMID: 21898777 Review.
-
A review on various aspects of organic synthesis using Comins' reagent.Mol Divers. 2022 Feb;26(1):691-716. doi: 10.1007/s11030-020-10175-2. Epub 2021 Jan 3. Mol Divers. 2022. PMID: 33389561 Review.
Cited by
-
Decarbonylative Sulfide Synthesis from Carboxylic Acids and Thioesters via Cross-Over C-S Activation and Acyl Capture.Org Chem Front. 2021 Sep 7;8(17):4805-4813. doi: 10.1039/d1qo00824b. Epub 2021 Jun 22. Org Chem Front. 2021. PMID: 34745635 Free PMC article.
-
Site-specific drug release of monomethyl fumarate to treat oxidative stress disorders.Nat Biotechnol. 2024 Nov 4:10.1038/s41587-024-02460-4. doi: 10.1038/s41587-024-02460-4. Online ahead of print. Nat Biotechnol. 2024. PMID: 39496929
-
Et3N/DMSO-supported one-pot synthesis of highly fluorescent β-carboline-linked benzothiophenones via sulfur insertion and estimation of the photophysical properties.Beilstein J Org Chem. 2020 Jul 20;16:1740-1753. doi: 10.3762/bjoc.16.146. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32765794 Free PMC article.
-
A Nonoxidative Sequence for the Preparation of 1,2-Diketone Derivatives Using Aldehyde and Organometallic Building Blocks.J Org Chem. 2022 Jun 17;87(12):8213-8222. doi: 10.1021/acs.joc.2c00439. Epub 2022 May 25. J Org Chem. 2022. PMID: 35613467 Free PMC article.
-
Chemoselective N-acylation of indoles using thioesters as acyl source.Beilstein J Org Chem. 2022 Jan 10;18:89-94. doi: 10.3762/bjoc.18.9. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35096177 Free PMC article.
References
-
- Bettòlo GBM, Casinovi CG, Galeffi C. A new class of quinones: sesquiterpenoid quinones of Mansonia Altissima. Tetrahedron Lett. 1965;52:4857–4864. doi: 10.1016/S0040-4039(01)89048-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

