Farm-like indoor microbiota in non-farm homes protects children from asthma development
- PMID: 31209334
- PMCID: PMC7617062
- DOI: 10.1038/s41591-019-0469-4
Farm-like indoor microbiota in non-farm homes protects children from asthma development
Erratum in
-
Author Correction: Farm-like indoor microbiota in non-farm homes protects children from asthma development.Nat Med. 2019 Aug;25(8):1319. doi: 10.1038/s41591-019-0546-8. Nat Med. 2019. PMID: 31316162
Abstract
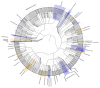
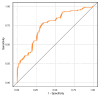
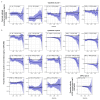
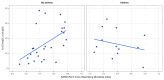
Asthma prevalence has increased in epidemic proportions with urbanization, but growing up on traditional farms offers protection even today1. The asthma-protective effect of farms appears to be associated with rich home dust microbiota2,3, which could be used to model a health-promoting indoor microbiome. Here we show by modeling differences in house dust microbiota composition between farm and non-farm homes of Finnish birth cohorts4 that in children who grow up in non-farm homes, asthma risk decreases as the similarity of their home bacterial microbiota composition to that of farm homes increases. The protective microbiota had a low abundance of Streptococcaceae relative to outdoor-associated bacterial taxa. The protective effect was independent of richness and total bacterial load and was associated with reduced proinflammatory cytokine responses against bacterial cell wall components ex vivo. We were able to reproduce these findings in a study among rural German children2 and showed that children living in German non-farm homes with an indoor microbiota more similar to Finnish farm homes have decreased asthma risk. The indoor dust microbiota composition appears to be a definable, reproducible predictor of asthma risk and a potential modifiable target for asthma prevention.
Conflict of interest statement
P.V.K, A.M.K., R.I.A., M.T., M.R., P.T., G.L., B.J., M.D., H.R., P.I.P., B.S., R.L., A.H., D.J.J.H. and J.P. do not have competing interests to disclose. M.J.E. and E.v.M. report patents EP2361632B1 and EP1964570B1 held by their institution LMU. E.v.M. reports recipient of funds from the European Commission for the conduct of the LUKAS (EFRAIM) and GABRIEL study and declares personal fees from Pharma Ventures, Peptinnovate Ltd., OM Pharma SA, European Commission/European Research Council Executive Agency, Tampereen Yliopisto, University of Turku, HAL Allergie GmbH, Ökosoziales Forum Oberösterreich and Mundipharma Deutschland GmbH & Co. KG; R.K. is a on the Scientific Advisory Board of Commense, Inc.
Figures





Comment in
-
Country-living in the city.Nat Rev Microbiol. 2019 Aug;17(8):462-463. doi: 10.1038/s41579-019-0234-1. Nat Rev Microbiol. 2019. PMID: 31253946 No abstract available.
-
Bauernhof-Hausstaub gegen Asthma : Kinder-Pneumologie.MMW Fortschr Med. 2020 Apr;162(6):32. doi: 10.1007/s15006-020-0329-7. MMW Fortschr Med. 2020. PMID: 32248482 German. No abstract available.
References
-
- von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nature reviews. Immunology. 2010;10:861–868. - PubMed
-
- Ege MJ, et al. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364:701–709. - PubMed
-
- Schuijs MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. - PubMed
-
- Karvonen AM, et al. Confirmed moisture damage at home, respiratory symptoms and atopy in early life: a birth-cohort study. Pediatrics. 2009;124:e329–338. - PubMed
-
- Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. Journal of Allergy and Clinical Immunology. 2015;136:860–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

