FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT
- PMID: 31209361
- PMCID: PMC7206111
- DOI: 10.1038/s41418-019-0372-z
FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT
Abstract
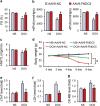
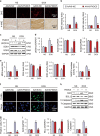
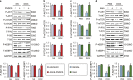
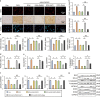
Oxidative stress and cardiomyocyte apoptosis play critical roles in doxorubicin (DOX)-induced cardiotoxicity. Previous studies indicated that fibronectin type III domain-containing 5 (FNDC5) and its cleaved form, irisin, could preserve mitochondrial function and attenuate oxidative damage as well as cell apoptosis, however, its role in DOX-induced cardiotoxicity remains unknown. Our present study aimed to investigate the role and underlying mechanism of FNDC5 on oxidative stress and cardiomyocyte apoptosis in DOX-induced cardiotoxicity. Cardiomyocyte-specific FNDC5 overexpression was achieved using an adeno-associated virus system, and then the mice were exposed to a single intraperitoneal injection of DOX (15 mg/kg) to generate DOX-induced cardiotoxicity. Herein, we found that FNDC5 expression was downregulated in DOX-treated murine hearts and cardiomyocytes. Fndc5 deficiency resulted in increased oxidative damage and apoptosis in H9C2 cells under basal conditions, imitating the phenotype of DOX-induced cardiomyopathy in vitro, conversely, FNDC5 overexpression or irisin treatment alleviated DOX-induced oxidative stress and cardiomyocyte apoptosis in vivo and in vitro. Mechanistically, we identified that FNDC5/Irisin activated AKT/mTOR signaling and decreased DOX-induced cardiomyocyte apoptosis, and moreover, we provided direct evidence that the anti-oxidant effect of FNDC5/Irisin was mediated by the AKT/GSK3β/FYN/Nrf2 axis in an mTOR-independent manner. And we also demonstrated that heat shock protein 20 was responsible for the activation of AKT caused by FNDC5/Irisin. In line with the data in acute model, we also found that FNDC5/Irisin exerted beneficial effects in chronic model of DOX-induced cardiotoxicity (5 mg/kg, i.p., once a week for three times, the total cumulative dose is 15 mg/kg) in mice. Based on these findings, we supposed that FNDC5/Irisin was a potential therapeutic agent against DOX-induced cardiotoxicity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85. - PubMed
-
- Silber JH, Barber G. Doxorubicin-induced cardiotoxicity. N Engl J Med. 1995;333:1359–60. - PubMed
-
- Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–42. - PubMed
-
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

