Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling
- PMID: 31209379
- PMCID: PMC6596419
- DOI: 10.1038/s41593-019-0419-y
Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling
Abstract
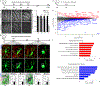
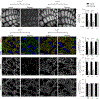
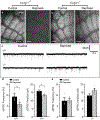
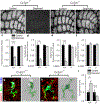
Microglia rapidly respond to changes in neural activity and inflammation to regulate synaptic connectivity. The extracellular signals, particularly neuron-derived molecules, that drive these microglial functions at synapses remain a key open question. Here we show that whisker lesioning, known to dampen cortical activity, induces microglia-mediated synapse elimination. This synapse elimination is dependent on signaling by CX3CR1, the receptor for microglial fractalkine (also known as CXCL1), but not complement receptor 3. Furthermore, mice deficient in CX3CL1 have profound defects in synapse elimination. Single-cell RNA sequencing revealed that Cx3cl1 is derived from cortical neurons, and ADAM10, a metalloprotease that cleaves CX3CL1 into a secreted form, is upregulated specifically in layer IV neurons and in microglia following whisker lesioning. Finally, inhibition of ADAM10 phenocopies Cx3cr1-/- and Cx3cl1-/- synapse elimination defects. Together, these results identify neuron-to-microglia signaling necessary for cortical synaptic remodeling and reveal that context-dependent immune mechanisms are utilized to remodel synapses in the mammalian brain.
Conflict of interest statement
Declaration of Interests
Authors declare no conflict of interest.
Figures



References
-
- Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

