Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats
- PMID: 31209877
- PMCID: PMC6713232
- DOI: 10.1113/JP278282
Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats
Abstract
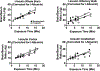
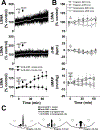
Key points: Pregnancy increases sympathetic nerve activity (SNA), although the mechanisms responsible for this remain unknown. We tested whether insulin or leptin, two sympathoexcitatory hormones increased during pregnancy, contribute to this. Transport of insulin across the blood-brain barrier in some brain regions, and into the cerebrospinal fluid (CSF), was increased, although brain insulin degradation was also increased. As a result, brain and CSF insulin levels were not different between pregnant and non-pregnant rats. The sympathoexcitatory responses to insulin and leptin were abolished in pregnant rats. Blockade of arcuate nucleus insulin receptors did not lower SNA in pregnant or non-pregnant rats. Collectively, these data suggest that pregnancy renders the brain resistant to the sympathoexcitatory effects of insulin and leptin, and that these hormones do not mediate pregnancy-induced sympathoexcitation. Increased muscle SNA stimulates glucose uptake. Therefore, during pregnancy, peripheral insulin resistance coupled with blunted insulin- and leptin-induced sympathoexcitation ensures adequate delivery of glucose to the fetus.
Abstract: Pregnancy increases basal sympathetic nerve activity (SNA), although the mechanism responsible for this remains unknown. Insulin and leptin are two sympathoexcitatory hormones that increase during pregnancy, yet, pregnancy impairs central insulin- and leptin-induced signalling. Therefore, to test whether insulin or leptin contribute to basal sympathoexcitation or, instead, whether pregnancy induces resistance to the sympathoexcitatory effects of insulin and leptin, we investigated α-chloralose anaesthetized late pregnant rats, which exhibited increases in lumbar SNA (LSNA), splanchnic SNA and heart rate (HR) compared to non-pregnant animals. In pregnant rats, transport of insulin into cerebrospinal fluid and across the blood-brain barrier in some brain regions increased, although brain insulin degradation was also increased; brain and cerebrospinal fluid insulin levels were not different between pregnant and non-pregnant rats. Although i.c.v. insulin increased LSNA and HR and baroreflex control of LSNA and HR in non-pregnant rats, these effects were abolished in pregnant rats. In parallel, pregnancy completely prevented the actions of leptin with respect to increasing lumbar, splanchnic and renal SNA, as well as baroreflex control of SNA. Blockade of insulin receptors (with S961) in the arcuate nucleus, the site of action of insulin, did not decrease LSNA in pregnant rats, despite blocking the effects of exogenous insulin. Thus, pregnancy is associated with central resistance to insulin and leptin, and these hormones are not responsible for the increased basal SNA of pregnancy. Because increases in LSNA to skeletal muscle stimulates glucose uptake, blunted insulin- and leptin-induced sympathoexcitation reinforces systemic insulin resistance, thereby increasing the delivery of glucose to the fetus.
Keywords: BBB insulin transport; S961; arcuate; insulin resistance; leptin; pregnancy; sympathetic nerve activity.
© 2019 The Authors. The Journal of Physiology © 2019 The Physiological Society.
Conflict of interest statement
Figures


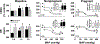

Similar articles
-
Obesity: sex and sympathetics.Biol Sex Differ. 2020 Mar 11;11(1):10. doi: 10.1186/s13293-020-00286-8. Biol Sex Differ. 2020. PMID: 32160920 Free PMC article. Review.
-
Sex differences in the sympathoexcitatory response to insulin in obese rats: role of neuropeptide Y.J Physiol. 2019 Mar;597(6):1757-1775. doi: 10.1113/JP277517. Epub 2019 Feb 3. J Physiol. 2019. PMID: 30628058 Free PMC article.
-
Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen.J Physiol. 2015 Apr 1;593(7):1633-47. doi: 10.1113/jphysiol.2014.284638. Epub 2014 Dec 22. J Physiol. 2015. PMID: 25398524 Free PMC article.
-
Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate.Hypertension. 2013 Apr;61(4):812-9. doi: 10.1161/HYPERTENSIONAHA.111.00518. Epub 2013 Feb 19. Hypertension. 2013. PMID: 23424232 Free PMC article.
-
Central angiotensin modulation of baroreflex control of renal sympathetic nerve activity in the rat: influence of dietary sodium.Acta Physiol Scand. 2003 Mar;177(3):285-9. doi: 10.1046/j.1365-201X.2003.01074.x. Acta Physiol Scand. 2003. PMID: 12608998 Review.
Cited by
-
Arcuate Angiotensin II Increases Arterial Pressure via Coordinated Increases in Sympathetic Nerve Activity and Vasopressin Secretion.eNeuro. 2022 Jan 20;9(1):ENEURO.0404-21.2021. doi: 10.1523/ENEURO.0404-21.2021. Print 2022 Jan-Feb. eNeuro. 2022. PMID: 34937769 Free PMC article.
-
Obesity: sex and sympathetics.Biol Sex Differ. 2020 Mar 11;11(1):10. doi: 10.1186/s13293-020-00286-8. Biol Sex Differ. 2020. PMID: 32160920 Free PMC article. Review.
-
Activation of nociception-sensitive ionotropic glutamate receptor-expressing rostroventrolateral medulla neurons by stimulation of cardiac afferents in rats.FASEB Bioadv. 2024 Aug 19;6(9):377-389. doi: 10.1096/fba.2024-00040. eCollection 2024 Sep. FASEB Bioadv. 2024. PMID: 39399474 Free PMC article.
-
Adaptations in autonomic nervous system regulation in normal and hypertensive pregnancy.Handb Clin Neurol. 2020;171:57-84. doi: 10.1016/B978-0-444-64239-4.00003-5. Handb Clin Neurol. 2020. PMID: 32736759 Free PMC article. Review.
-
Evidence for an alternative insulin transporter at the blood-brain barrier.Aging Pathobiol Ther. 2022;4(4):100-108. doi: 10.31491/apt.2022.12.100. Epub 2022 Dec 29. Aging Pathobiol Ther. 2022. PMID: 36644126 Free PMC article.
References
-
- Anglin JC & Brooks VL. (2003). Tyrosine hydroxylase and norepinephrine transporter in sympathetic ganglia of female rats vary with reproductive state. AutonNeurosci 105, 8–15. - PubMed
-
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R & Morley JE. (2004). Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53, 1253–1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

