Optimization of formulation for enhanced intranasal delivery of insulin with translationally controlled tumor protein-derived protein transduction domain
- PMID: 31210056
- PMCID: PMC6586149
- DOI: 10.1080/10717544.2019.1628119
Optimization of formulation for enhanced intranasal delivery of insulin with translationally controlled tumor protein-derived protein transduction domain
Abstract
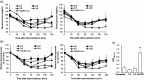
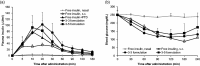
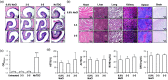
Intranasal delivery of insulin is an alternative approach to treat diabetes, as it enables higher patient compliance than conventional therapy with subcutaneously injected insulin. However, the use of intranasal delivery of insulin is limited for insulin's hydrophilicity and vulnerability to enzymatic degradation. This limitation makes optimization of formulation intranasal insulin for commercial purpose indispensable. This study evaluated bioavailability (BA) of various formulations of insulin intranasally delivered with protein transduction domain (PTD) derived from translationally controlled tumor protein. The therapeutic efficacy of newly formulated intranasal insulin + PTD was compared in vivo studies with normal and alloxan-induced diabetic rats, to those of free insulin and subcutaneously injected insulin. BA of insulin in two new formulations was, respectively, 60.71% and 45.81% of subcutaneously injected insulin, while the BA of free insulin was only 3.34%. Histological analysis of tissues, lactate dehydrogenase activity in nasal fluid, and biochemical analysis of sera revealed no detectable topical or systemic toxicity in rats and mice. Furthermore, stability analysis of newly formulated insulin + PTD to determine the optimal conditions for storage revealed that when stored at 4 °C, the delivery capacity of insulin was maintained up to 7 d. These results suggest that the new formulations of intranasal insulin are suitable for use in diabetes therapy and are easier to administer.
Keywords: Drug formulation; insulin; intranasal delivery; protein transduction domain; translationally controlled tumor protein.
Figures

References
-
- Arora P, Sharma S, Garg S (2002). Permeability issues in nasal drug delivery. Drug Discov Today 7:967–75. - PubMed
-
- Bae HD, Lee K (2013). On employing a translationally controlled tumor protein-derived protein transduction domain analog for transmucosal delivery of drugs. J Control Release 170:358–64. - PubMed
-
- Bae H-D, Lee J, Jin X-H, et al. (2016). Potential of translationally controlled tumor protein-derived protein transduction domains as antigen carriers for nasal vaccine delivery. Mol Pharm 13:3196–205. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
