Artemether for severe malaria
- PMID: 31210357
- PMCID: PMC6580442
- DOI: 10.1002/14651858.CD010678.pub3
Artemether for severe malaria
Abstract
Background: In 2011 the World Health Organization (WHO) recommended parenteral artesunate in preference to quinine as first-line treatment for people with severe malaria. Prior to this recommendation many countries, particularly in Africa, had begun to use artemether, an alternative artemisinin derivative. This Cochrane Review evaluates intramuscular artemether compared with both quinine and artesunate.
Objectives: To assess the efficacy and safety of intramuscular artemether versus any other parenteral medication in the treatment of severe malaria in adults and children.
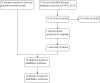
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (the Cochrane Library), MEDLINE, Embase, and LILACS, ISI Web of Science, conference proceedings, and reference lists of articles. We also searched the WHO International Clinical Trial Registry Platform, ClinicalTrials.gov, and the metaRegister of Controlled Trials (mRCT) for ongoing trials up to 7 September 2018. We checked the reference lists of all studies identified by the search. We examined references listed in review articles and previously compiled bibliographies to look for eligible studies.
Selection criteria: Randomized controlled trials (RCTs) comparing intramuscular artemether with intravenous/intramuscular quinine or artesunate for treating severe malaria.
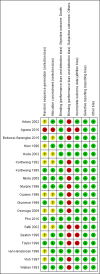
Data collection and analysis: The primary outcome was all-cause death. Two review authors independently screened each article by title and abstract, and examined potentially relevant studies for inclusion using an eligibility form. Two review authors independently extracted data and assessed risk of bias of included studies. We summarized dichotomous outcomes using risk ratios (RRs) and continuous outcomes using mean differences (MDs), and have presented both measures with 95% confidence intervals (CIs). Where appropriate, we combined data in meta-analyses and used the GRADE approach to summarize the certainty of the evidence.
Main results: We included 19 RCTs, enrolling 2874 adults and children with severe malaria, carried out in Africa (12 trials) and in Asia (7 trials).Artemether versus quinineFor children, there is probably little or no difference in the risk of death between intramuscular artemether and quinine (RR 0.97, 95% CI 0.77 to 1.21; 13 trials, 1659 participants, moderate-certainty evidence). Coma resolution time may be about five hours shorter with artemether (MD -5.45, 95% CI -7.90 to -3.00; six trials, 358 participants, low-certainty evidence). Artemether may make little difference to neurological sequelae (RR 0.84, 95% CI 0.66 to 1.07; seven trials, 968 participants, low-certainty evidence). Compared to quinine, artemether probably shortens the parasite clearance time by about nine hours (MD -9.03, 95% CI -11.43 to -6.63; seven trials, 420 participants, moderate-certainty evidence), and may shorten the fever clearance time by about three hours (MD -3.73, 95% CI -6.55 to -0.92; eight trials, 457 participants, low-certainty evidence).For adults, treatment with intramuscular artemether probably results in fewer deaths than treatment with quinine (RR 0.59, 95% CI 0.42 to 0.83; four trials, 716 participants, moderate-certainty evidence).Artemether versus artesunateArtemether and artesunate have not been directly compared in randomized trials in children.For adults, mortality is probably higher with intramuscular artemether (RR 1.80, 95% CI 1.09 to 2.97; two trials, 494 participants, moderate-certainty evidence).
Authors' conclusions: Artemether appears to be more effective than quinine in children and adults. Artemether compared to artesunate has not been extensively studied, but in adults it appears inferior. These findings are consistent with the WHO recommendations that artesunate is the drug of choice, but artemether is acceptable when artesunate is not available.
Conflict of interest statement
EE has no known conflicts of interest. EEE has no known conflicts of interest. OO has no known conflicts of interest. MM has no known conflicts of interest.
Figures
Update of
-
Artemether for severe malaria.Cochrane Database Syst Rev. 2014 Sep 11;2014(9):CD010678. doi: 10.1002/14651858.CD010678.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Jun 18;6:CD010678. doi: 10.1002/14651858.CD010678.pub3. PMID: 25209020 Free PMC article. Updated.
References
References to studies included in this review
Adam 2002 {published data only}
-
- Adam I, Idris HM, Mohamed‐Ali AA, Aelbasit IA, Elbashir MI. Comparison of intramuscular artemether and intravenous quinine in the treatment of Sudanese children with severe falciparum malaria. East African Medical Journal 2002;79(12):621‐5. - PubMed
Aguwa 2010 {published data only}
-
- Aguwa CN, Ukwe CV, Adibe MO. A comparative study of quinine and artemether in the treatment of severe malaria in Nigerian children. Tropical Journal of Pharmaceutical Research 2010;9(1):11‐7.
Bobossi‐Serengbe 2015 {published data only}
-
- Bobossi‐Serengbe G, Gody JC, Fioboy R, Elowa JB, Manirakiza A. Comparison of the effectiveness of artemether and quinine for treatment of severe malaria in children, Bangui, Central African Republic. Bulletin de la Societe de Pathologie Exotique (1990) 2015;108(2):107‐11. - PubMed
Hien 1996 {published data only}
-
- Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. New England Journal of Medicine 1996;335(2):76‐83. - PubMed
Huda 2003 {published data only}
-
- Huda SN, Shahab T, Ali SM, Afzal K, Khan HM. A comparative clinical trial of artemether and quinine in children with severe malaria. Indian Pediatrics 2003;40(10):939‐45. - PubMed
Karbwang 1992 {published data only}
-
- Karbwang J, Sukontason K, Rimchala W, Namsiripongpun W, Tin T, Auprayoon P, et al. Preliminary report: a comparative clinical trial of artemether and quinine in severe falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):768‐72. - PubMed
Karbwang 1995 {published data only}
-
- Karbwang J, Tin T, Rimchala W, Sukontason K, Namsiripongpun V, Thanavibul A, et al. Comparison of artemether and quinine in the treatment of severe falciparum malaria in south‐east Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(6):668‐71. - PubMed
Minta 2005 {published data only}
-
- Minta D, Sissoko M, Sidibe I, Dolo A, Poudiougou B, Dembele M, et al. Efficacy and safety of artemether in the treatment of severe and complicated malaria in Mali [Efficacite et tolerance de l'artemether dans le traitement du paludisme grave et complique au Mali]. Mali Médical 2005;20(1‐2):28‐32. - PubMed
Murphy 1996 {published data only}
-
- Murphy S, English M, Waruiru C, Mwangi I, Amukoye E, Crawley J, et al. An open randomized trial of artemether versus quinine in the treatment of cerebral malaria in African children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(3):298‐301. - PubMed
Ojuawo 1998 {published data only}
-
- Ojuawo A, Adegboye AR, Oyewalo O. Clinical response and parasite clearance in childhood cerebral malaria: A comparison between intramuscular artemether and intravenous quinine. East African Medical Journal 1998;75:450‐2.
Olumese 1999 {published data only}
-
- Olumese PE, Björkman A, Gbadegesin RA, Adeyemo AA, Walker O. Comparative efficacy of intramuscular artemether and intravenous quinine in Nigerian children with cerebral malaria. Acta Tropica 1999;73(3):231‐6. - PubMed
Osonuga 2009 {published data only}
-
- Osonuga O, Osonuga AA, Osonuga IO, Osonuga A. Temperature changes in the course of treatment of severe malaria patients with artemether and quinine. Asian Journal of Medical Sciences 2011;2(1):41‐5.
-
- Osonuga OA, Osonuga AA, Osonuga IO, Osonuga A. Comparison of Coma Resolution Time in the Course of Treating Childrenwith Severe Falciparum Malaria with Quinine and Artemether. World Journal of Medical Sciences 2011;6(2):42‐5.
-
- Osonuga OA, Osonuga IO. Parasitaemia changes in the course of treatment of severe malaria patients with artemether and quinine (A preliminary study). Macedonian Journal of Medical Sciences 2009;2(4):319‐23.
Phu 2010 {published data only}
Satti 2002 {published data only}
-
- Satti GM, Elhassan SH, Ibrahim SA. The efficacy of artemether versus quinine in the treatment of cerebral malaria. Journal of the Egyptian Society of Parasitology 2002;32(2):611‐23. - PubMed
Seaton 1998 {published data only}
-
- Seaton RA, Trevett AJ, Wembri JP, Nwokolo N, Naraqi S, Black J, et al. Randomized comparison of intramuscular artemether and intravenous quinine in adult, Melanesian patients with severe or complicated, Plasmodium falciparum malaria in Papua New Guinea. Annals of Tropical Medicine and Parasitology 1998;92(2):133‐9. - PubMed
Taylor 1998 {published data only}
-
- Taylor TE, Wills BA, Courval JM, Molyneux ME. Intramuscular artemether vs intravenous quinine: an open, randomized trial in Malawian children with cerebral malaria. Tropical Medicine and International Health 1998;3(1):3‐8. - PubMed
van Hensbroek 1996 {published data only}
-
- Hensbroek MB, Onyiorah E, Jaffar S, Schneider G, Palmer A, Frenkel J, et al. A trial of artemether or quinine in children with cerebral malaria. New England Journal of Medicine 1996;335(2):69‐75. - PubMed
Vinh 1997 {published data only}
-
- Ha V, Nguyen NH, Tran TB, Bui MC, Nguyen HP, Tran TH, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):465‐7. - PubMed
Walker 1993 {published data only}
-
- Walker O, Salako LA, Omukhodion SI, Sowunmi A. An open randomized comparative study of intramuscular artemether and intravenous quinine in cerebral malaria in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):564‐6. - PubMed
References to studies excluded from this review
Aceng 2005 {published data only}
Bhattacharya 1997 {published data only}
-
- Bhattacharya PC, Pai‐dhungat AJ, Patel K. Artemether in moderate to severe malaria: a multicenter trial in India. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(4):736‐40. - PubMed
Bunnag 1992 {published data only}
-
- Bunnag D, Karbwang J, Harinasuta T. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):762‐7. - PubMed
Dunyo 2011 {published data only}
Falade 2007 {published data only}
-
- Falade CO, Fadero FF, Happi CT, Dada‐Adegbola HO, Gbotosho GO, Ayede I, et al. Dihydroartemisinin suppository in moderately severe malaria: comparative efficacy of dihydroartemisinin suppository versus intramuscular artemether followed by oral sulfadoxine‐pyrimethamine in the management of moderately severe malaria in Nigerian children. American Journal of Tropical Medicine and Hygiene 2007;76(1):1‐6. - PubMed
Fargier 1999 {published data only}
-
- Fargier JJ, Louis FJ, Duparc S, Hounsinou C, Ringwald P, Danis M. Comparative study of artemether and quinine in severe Plasmodium falciparum malaria in adults and older children in Cameroon. Médicine Tropicale 1999;59(2):151‐6. - PubMed
Karbwang 1994 {published data only}
-
- Karbwang J, Na‐Bangchang K, Wattanakoon Y, Thanavibul A, Harinasuta T. Artemether 5 versus 7 day regimen for severe falciparum malaria. Southeast Asian Journal ofTropical Medicine and Public Health 1994;25(4):702‐6. - PubMed
Karunajeewa 2006 {published data only}
Myint 1987 {published data only}
-
- Myint PT, Shwe T. A controlled clinical trial of artemether (qinghaosu derivative) versus quinine in complicated and severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(4):559‐61. - PubMed
Osonuga 2006 {published data only}
-
- Osonuga OA, Osonuga IO, Akinsomisoye OS, Raji Y, Ademowo OG. Packed cell volume changes in the treatment of severe malarial patients with artemether and quinine (a preliminary study). Journal of Medical Sciences 2006;6(5):853‐7.
Reham 2012 {published data only}
-
- Rehman HU, Bilal H, Ali Q. Comparative study of intramuscular artemether and intravenous quinine in the treatment of cerebral malaria in children. Pakistan Paediatric Journal 2012;36(2):75‐80.
Rehman 2013 {published data only}
-
- Rehman MU, Shrestha B, Zehri T, Thapa S. Efficacy of quinine versus artemether in the treatment of severe malaria. Journal of the Nepal Health Research Council 2013;11(23):17‐21. - PubMed
Shwe 1988 {published data only}
-
- Tin Shwe, Pe Than Myint, Ye Htut, Win Myint, Lin Soe. The effect of mefloquine artemether compared with quinine on patients with complicated falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(5):665‐6. - PubMed
Shwe 1992 {published data only}
-
- Shwe T, Hla KK. The effect of artemether plus mefloquine on Myanmar patients with complicated falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(Suppl 4):117‐21. - PubMed
White 1992 {published data only}
-
- White NJ, Waller D, Crawley J, Nosten F, Chapman D, Brewster D, et al. Comparison of artemether and chloroquine for severe malaria in Gambian children. Lancet 1992;339(8789):317‐21. - PubMed
References to studies awaiting assessment
Danis 1996 {published data only}
-
- Danis M, Chandenier J, Doumbo O, Kombila M, Kouame J, Louis F, et al. Results obtained with i.m. artemether versus i.v. quinine in the treatment of severe malaria in a multi‐centre study in Africa. Japan Journal of Tropical Medicine and Hygiene 1996;24(Suppl 1):93‐6.
Faiz 2001 {published data only}
-
- Faiz MA, Rahman E, Hossain MA, Rahman MR, Yunus EB, Samad R, et al. A randomized controlled trial comparing artemether and quinine in the treatment of cerebral malaria in Bangladesh. Indian Journal of Malariology 2001;38(1‐2):9‐18. - PubMed
Additional references
Adjuik 2004
-
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, WhiteN. International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. Lancet 2004;363(9402):9–17. - PubMed
AQMSG 2001
-
- Artemether‐Quinine Meta‐analysis Study Group. A meta‐analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(6):637‐50. - PubMed
Ashley 2014
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Cui 2015
Dondorp 2005
-
- Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005;366(9487):717‐25. - PubMed
Dondorp 2010
Doolan 2009
Guyatt 2008
Hawkes 2014
-
- Hawkes M, Conroy AL, Kain KC. Spread of artemisinin resistance in malaria. New England Journal of Medicine 2014;371(20):1944–5. - PubMed
Hien 2004
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Illet 2002
Jaffar 1997
-
- Jaffar S, Hensbroek M, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. American Journal of Tropical Medicine and Hygiene 1997;57(1):20–4. - PubMed
Karbwang 1997
-
- Karbwang J, Na‐Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. European Journal of Clinical Pharmacology 1997;52(4):307–10. - PubMed
Kyu 2009
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McIntosh 2000
Menard 2016
Mithwani 2004
-
- Mithwani S, Aarons L, Kokwaro GO, Majid O, Muchohi S, Edwards G, et al. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria. British Journal of Clinical Pharmacology 2004;57(2):146–52. - PMC - PubMed
Murphy 1997
-
- Murphy SA, Mberu E, Muhia D, English M, Crawley J, Waruiru C, et al. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):331‐4. - PubMed
Navaratnam 2000
-
- Navaratnam V, Mansor SM, Sit NW, Grace J, Li Q, Olliaro P. Pharmacokinetics of artemisinin‐type compounds. Clinical Pharmacokinetics 2000;39(4):255‐70. - PubMed
Nealon 2002
Pittler 1999
-
- Pittler MH, Ernst E. Artemether for severe malaria: a meta‐analysis of randomized clinical trials. Clinical Infectious Diseases 1999;28(3):597‐601. - PubMed
PrayGod 2008
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sinclair 2012
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
ter Kuile 1993
-
- ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Experimental Parasitology 1993;76(1):85–95. - PubMed
Tyagi 2018
WHO 2000
-
- World Health Organization. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(Suppl 1):S1–90. - PubMed
WHO 2008
-
- World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization, 2008.
WHO 2010
-
- World Health Organization. Roll Back Malaria Department. Guidelines for the treatment of malaria. Second Edition. Geneva: World Health Organization, 2010.
WHO 2015
-
- World Health Organization. Roll Back Malaria Department. Guidelines for the Treatment of Malaria. Third Edition. Geneva: World Health Organization, 2015.
Wongsrichanalai 2002
-
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug‐resistant malaria. The Lancet infectious diseases 2002;2(4):209‐18. - PubMed
References to other published versions of this review
Esu 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous