A novel myeloid cell in murine spleen defined through gene profiling
- PMID: 31210415
- PMCID: PMC6653018
- DOI: 10.1111/jcmm.14382
A novel myeloid cell in murine spleen defined through gene profiling
Abstract
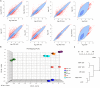
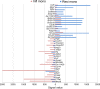
A novel myeloid antigen presenting cell can be generated through in vitro haematopoiesis in long-term splenic stromal cocultures. The in vivo equivalent subset was recently identified as phenotypically and functionally distinct from the spleen subsets of macrophages, conventional (c) dendritic cells (DC), resident monocytes, inflammatory monocytes and eosinophils. This novel subset which is myeloid on the basis of cell surface phenotype, but dendritic-like on the basis of cell surface marker expression and antigen presenting function, has been tentatively labelled "L-DC." Transcriptome analysis has now been employed to determine the lineage relationship of this cell type with known splenic cDC and monocyte subsets. Principal components analysis showed separation of "L-DC" and monocytes from cDC subsets in the second principal component. Hierarchical clustering then indicated a close lineage relationship between this novel subset, resident monocytes and inflammatory monocytes. Resident monocytes were the most closely aligned, with no genes specifically expressed by the novel subset. This subset, however, showed upregulation of genes reflecting both dendritic and myeloid lineages, with strong upregulation of several genes, particularly CD300e. While resident monocytes were found to be dependent on Toll-like receptor signalling for development and were reduced in number in Myd88-/- and Trif-/- mutant mice, both the novel subset and inflammatory monocytes were unaffected. Here, we describe a novel myeloid cell type closely aligned with resident monocytes in terms of lineage but distinct in terms of development and functional capacity.
Keywords: dendritic cells; monocytes; myelopoiesis; spleen.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Antigen presenting capacity of murine splenic myeloid cells.BMC Immunol. 2017 Jan 11;18(1):4. doi: 10.1186/s12865-016-0186-4. BMC Immunol. 2017. PMID: 28077081 Free PMC article.
-
Haematopoietic stem cells in spleen have distinct differentiative potential for antigen presenting cells.J Cell Mol Med. 2010 Aug;14(8):2144-50. doi: 10.1111/j.1582-4934.2009.00923.x. Epub 2010 Oct 3. J Cell Mol Med. 2010. PMID: 19799644 Free PMC article.
-
Hematopoiesis of immature myeloid dendritic cells in stroma-dependent spleen long-term cultures occurs independently of NF-KB/RelB function.Exp Hematol. 2007 Oct;35(10):1580-93. doi: 10.1016/j.exphem.2007.07.007. Exp Hematol. 2007. PMID: 17889723
-
In vitro hematopoiesis reveals a novel dendritic-like cell present in murine spleen.Curr Stem Cell Res Ther. 2013 Sep;8(5):365-9. doi: 10.2174/1574888x113089990050. Curr Stem Cell Res Ther. 2013. PMID: 23971833 Review.
-
In vitro haematopoiesis of a novel dendritic-like cell present in murine spleen.Curr Stem Cell Res Ther. 2010 Dec;5(4):367-71. doi: 10.2174/157488810793351749. Curr Stem Cell Res Ther. 2010. PMID: 20528761 Review.
Cited by
-
High-throughput analysis of lung immune cells in a combined murine model of agriculture dust-triggered airway inflammation with rheumatoid arthritis.PLoS One. 2021 Feb 12;16(2):e0240707. doi: 10.1371/journal.pone.0240707. eCollection 2021. PLoS One. 2021. PMID: 33577605 Free PMC article.
-
Single cell RNA-seq reveals cellular and transcriptional heterogeneity in the splenic CD11b+Ly6Chigh monocyte population expanded in sepsis-surviving mice.Mol Med. 2024 Nov 6;30(1):202. doi: 10.1186/s10020-024-00970-0. Mol Med. 2024. PMID: 39506629 Free PMC article.
References
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193‐197. - PubMed
-
- Kita K, Nakase K, Miwa H, et al. Phenotypical characteristics of acute myelocytic leukemia associated with the t(8;21)(q22;q22) chromosomal abnormality: frequent expression of immature B‐cell antigen CD19 together with stem cell antigen CD34. Blood. 1992;80:470‐477. - PubMed
-
- Shortman K, Naik SH. Steady‐state and inflammatory dendritic‐cell development. Nat Rev Immunol. 2007;7:19‐30. - PubMed
-
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978‐2986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

