Assessment of Short-Term Engraftment Potential of Ex Vivo Expanded Hematopoietic Stem Cells Using Normal Fetal Mouse in Utero Transplantation Model
- PMID: 31210431
- PMCID: PMC6582413
- DOI: 10.22074/cellj.2019.6006
Assessment of Short-Term Engraftment Potential of Ex Vivo Expanded Hematopoietic Stem Cells Using Normal Fetal Mouse in Utero Transplantation Model
Abstract
Objective: Ex vivo expansion is a promising strategy to overcome the low number of human umbilical cord blood hematopoietic stem cells (hUCB-HSCs). Although based on the obtained results in unnatural physiological condition of irradiated genetically immune-deficient mouse models, there has always been concern that the expanded cells have less engraftment potential. The purpose of this study was to investigate effect of common ex vivo expansion method on engraftment potential of hUCB-mononuclear cells (MNCs), using normal fetal mouse, as a model with more similarity to human physiological conditions.
Materials and methods: In this experimental study, briefly, isolated hUCB-MNCs were cultured in common expansion medium containing stem cell factor, Flt3 ligand and thrombopoietin. The unexpanded and expanded cells were transplanted to the fetal mice on gestational days of 11.5-13.5. After administration of human hematopoiesis growth factors (hHGFs), presence of human CD45+ cells, in the peripheral blood of recipients, was assessed at various time points after transplantation.
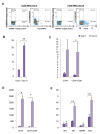
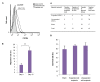
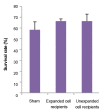
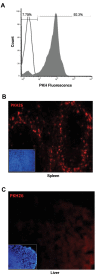
Results: The expanded MNCs showed 32-fold increase in the expression of CD34+38- phenotype and about 3-fold higher clonogenic potential as compared to the uncultured cells. Four weeks after transplantation, 73% (19/26) of expanded-cell recipients and 35% (7/20) of unexpanded-cell recipients were found to be successfully engrafted with human CD45+ cells. The engraftment level of expanded MNCs was significantly (1.8-fold) higher than unexpanded cells. After hHGFs administration, the level was increased to 3.2, 3.8 and 2.6-fold at respectively 8, 12, and 16 weeks of post transplantation. The increased expression of CXCR4 protein in expanded MNCs is a likely explanation for the present findings.
Conclusion: The presented data showed that expanded MNCs compared to unexpended cells are capable of more rapid and higher short-term engraftment in normal fetal mouse. It could also be suggested that in utero transplantation (IUT) of normal fetal mice could be an appropriate substitute for NOD/SCID mice in xenotransplantation studies.
Keywords: Chimerism; Cord Blood Stem Cell Transplantation; Hematopoietic Stem Cells.
Copyright© by Royan Institute. All rights reserved.
Conflict of interest statement
There is no conflict of interest in this study.
Figures

Similar articles
-
Delayed engraftment of nonobese diabetic/severe combined immunodeficient mice transplanted with ex vivo-expanded human CD34(+) cord blood cells.Blood. 1999 Feb 1;93(3):1097-105. Blood. 1999. PMID: 9920860
-
Umbilical cord blood progeny cells that retain a CD34+ phenotype after ex vivo expansion have less engraftment potential than unexpanded CD34+ cells.Transfusion. 2001 Feb;41(2):213-8. doi: 10.1046/j.1537-2995.2001.41020213.x. Transfusion. 2001. PMID: 11239225
-
Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential.Exp Hematol. 2002 Jun;30(6):612-6. doi: 10.1016/s0301-472x(02)00805-6. Exp Hematol. 2002. PMID: 12063029
-
Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice.Ann N Y Acad Sci. 2001 Jun;938:9-17. doi: 10.1111/j.1749-6632.2001.tb03569.x. Ann N Y Acad Sci. 2001. PMID: 11458530 Review.
-
Functional characterization and therapeutic potential of human umbilical cord blood mononuclear cells.Regen Ther. 2024 Dec 6;28:101-114. doi: 10.1016/j.reth.2024.11.019. eCollection 2025 Mar. Regen Ther. 2024. PMID: 40166041 Free PMC article. Review.
Cited by
-
Combination of SB431542, Chir9901, and Bpv as a novel supplement in the culture of umbilical cord blood hematopoietic stem cells.Stem Cell Res Ther. 2020 Nov 9;11(1):474. doi: 10.1186/s13287-020-01945-8. Stem Cell Res Ther. 2020. PMID: 33168035 Free PMC article.
References
-
- Chabannon C, Kuball J, Bondanza A, Dazzi F, Pedrazzoli P, Toubert A, et al. Hematopoietic stem cell transplantation in its 60s: A platform for cellular therapies. Sci Transl Med. 2018;10(436) - PubMed
-
- Hough R, Danby R, Russell N, Marks D, Veys P, Shaw B, et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br J Haematol. 2016;172(3):360–370. - PubMed
-
- Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43(7):498–513. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
