The Common Rhythm of Action and Perception
- PMID: 31210564
- PMCID: PMC6938410
- DOI: 10.1162/jocn_a_01436
The Common Rhythm of Action and Perception
Abstract
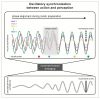
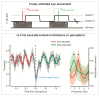
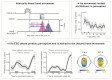
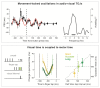
Research in the last decade has undermined the idea of perception as a continuous process, providing strong empirical support for its rhythmic modulation. More recently, it has been revealed that the ongoing motor processes influence the rhythmic sampling of sensory information. In this review, we will focus on a growing body of evidence suggesting that oscillation-based mechanisms may structure the dynamic interplay between the motor and sensory system and provide a unified temporal frame for their effective coordination. We will describe neurophysiological data, primarily collected in animals, showing phase-locking of neuronal oscillations to the onset of (eye) movements. These data are complemented by novel evidence in humans, which demonstrate the behavioral relevance of these oscillatory modulations and their domain-general nature. Finally, we will discuss the possible implications of these modulations for action-perception coupling mechanisms.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

