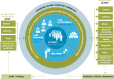
Registry stakeholders
- PMID: 31210971
- PMCID: PMC6549107
- DOI: 10.1302/2058-5241.4.180077
Registry stakeholders
Abstract
Clinical registries are health information systems, which have the mission to collect multidimensional real-world data over the long term, and to generate relevant information and actionable knowledge to address current serious healthcare problems.This article provides an overview of clinical registries and their relevant stakeholders, focussing on registry structure and functioning, each stakeholder's specific interests, and on their involvement in the registry's information input and output.Stakeholders of clinical registries include the patients, healthcare providers (professionals and facilities), financiers (government, insurance companies), public health and regulatory agencies, industry, the research community and the media.The article discusses (1) challenges in stakeholder interaction and how to strengthen the central role of the patient, (2) the importance of adding cost reporting to enable informed value choices, and (3) the need for proof of clinical and public health utility of registries.In its best form, a registry is a mission-driven, independent stakeholder-registry team collaboration that enables rapid, transparent and open-access knowledge generation and dissemination. Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180077.
Keywords: health information system; registry; stakeholder.
Conflict of interest statement
ICMJE Conflict of interest statement: PH declares grants from Fondation pour la recherche ostéoarticulaire. He is a board member of Fondation pour la recherche ostéoarticulaire and the Swiss Foundation for Innovation and Training in Surgery (SFITS), and he is the Editor-in-Chief of EFORT Open Reviews. AL is an editorial board member of EFORT Open Reviews and a board member of Fondation pour la recherche ostéoarticulaire. AJC has nothing to declare.
Figures
References
-
- Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for evaluating patient outcomes: a user’s guide. Third ed. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [Internet] - PubMed
-
- Olsen LA, Aisner D, McGinnis JM, eds. The learning healthcare system: workshop summary. Institute of Medicine (IOM) Roundtable on Evidence-Based Medicine. Washington, DC: National Academies Press, 2007 - PubMed
-
- Lehoux P, Williams-Jones B, Miller F, Urbach D, Tailliez S. What leads to better health care innovation? Arguments for an integrated policy-oriented research agenda. J Health Serv Res Policy 2008;13:251–254. - PubMed
Publication types
LinkOut - more resources
Full Text Sources