Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke
- PMID: 31211345
- PMCID: PMC6582268
- DOI: 10.1001/jama.2019.7525
Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke
Abstract
Importance: Reducing postprocedural stroke is important to improve the safety of transcatheter aortic valve replacement (TAVR).
Objective: This study evaluated the trends of stroke occurring within 30 days after the procedure during the first 5 years TAVR was used in the United States, the association of stroke with 30-day mortality, and the association of medical therapy with 30-day stroke risk.
Design, setting, and participants: Retrospective cohort study including 101 430 patients who were treated with femoral and nonfemoral TAVR at 521 US hospitals in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry from November 9, 2011, through May 31, 2017. Thirty-day follow-up ended June 30, 2017.
Exposures: TAVR.
Main outcomes and measures: The rates of 30-day transient ischemic attack and stroke were assessed. Association of stroke with 30-day mortality and association of antithrombotic medical therapies with postdischarge 30-day stroke were assessed with a Cox proportional hazards model and propensity-score matching, respectively.
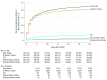
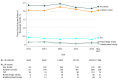
Results: Among 101 430 patients included in the study (median age, 83 years [interquartile range {IQR}, 76-87 years]; 47 797 women [47.1%]; and 85 147 patients [83.9%] treated via femoral access), 30-day postprocedure follow-up data was assessed in all patients. At day 30, there were 2290 patients (2.3%) with a stroke of any kind (95% CI, 2.2%-2.4%), and 373 patients (0.4%) with transient ischemic attacks (95% CI, 0.3%-0.4%) . During the study period, 30-day stroke rates were stable without an increasing or decreasing trend in all patients (P for trend = .22) and in the large femoral access subgroup (P trend = .47). Among cases of stroke within 30 days, 1119 strokes (48.9%) occurred within the first day and 1567 (68.4%) within 3 days following TAVR. The occurrence of stroke was associated with a significant increase in 30-day mortality: 383 patients (16.7%) of 2290 who had a stroke vs 3662 patients (3.7%) of 99 140 who did not have a stroke died (P < .001; risk-adjusted hazard ratio [HR], 6.1 [95% CI, 5.4-6.8]; P < .001). After propensity-score matching, 30-day stroke risk was not associated with whether patients in the femoral cohort were (0.55%) or were not (0.52%) treated with dual antiplatelet therapy at hospital discharge (HR, 1.04; 95% CI, 0.74-1.46) nor was it associated with whether patients in the nonfemoral cohort were (0.71%) or were not (0.69%) treated with dual antiplatelet therapy (HR, 1.02; 95% CI, 0.54-1.95). Similarly, 30-day stroke risk was not associated with whether patients in the femoral cohort were (0.57%) or were not (0.55) treated with oral anticoagulant therapy at hospital discharge (HR, 1.03; 95% CI, 0.73-1.46) nor was it associated with whether patients in the nonfemoral cohort were (0.75%) or were not (0.82%) treated with an oral anticoagulant (HR, 0.93; 95% CI, 0.47-1.83).
Conclusions and relevance: Between 2011 and 2017, the rate of 30-day stroke following transcatheter aortic valve replacement in a US registry population remained stable.
Conflict of interest statement
Figures

Comment in
-
Stroke After Transcatheter Aortic Valve Replacement: An Important but Underreported Outcome in Clinical Practice.JAMA. 2019 Jun 18;321(23):2287-2289. doi: 10.1001/jama.2019.8613. JAMA. 2019. PMID: 31211329 No abstract available.
Similar articles
-
Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke.JAMA. 2019 Jun 11;321(22):2193-2202. doi: 10.1001/jama.2019.7108. JAMA. 2019. PMID: 31184741 Free PMC article.
-
Clinical outcomes at 1 year following transcatheter aortic valve replacement.JAMA. 2015 Mar 10;313(10):1019-28. doi: 10.1001/jama.2015.1474. JAMA. 2015. PMID: 25756438
-
Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk.JAMA. 2021 Sep 21;326(11):1034-1044. doi: 10.1001/jama.2021.13346. JAMA. 2021. PMID: 34546301 Free PMC article.
-
Meta-Analysis of Stroke and Mortality Rates in Patients Undergoing Valve-in-Valve Transcatheter Aortic Valve Replacement.J Am Heart Assoc. 2021 Mar 16;10(6):e019512. doi: 10.1161/JAHA.120.019512. Epub 2021 Mar 8. J Am Heart Assoc. 2021. PMID: 33682426 Free PMC article.
-
Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: A meta-analysis of randomized trials and propensity score matched observational studies.Catheter Cardiovasc Interv. 2018 Aug 1;92(2):408-416. doi: 10.1002/ccd.27518. Epub 2018 Feb 1. Catheter Cardiovasc Interv. 2018. PMID: 29388308
Cited by
-
Comparison of latest generation supra-annular and intra-annular self-expanding transcatheter heart valves.J Thorac Dis. 2020 Nov;12(11):6769-6779. doi: 10.21037/jtd-20-1700. J Thorac Dis. 2020. PMID: 33282378 Free PMC article.
-
What Are SAVR Indications in the TAVI Era?J Clin Med. 2025 Mar 29;14(7):2357. doi: 10.3390/jcm14072357. J Clin Med. 2025. PMID: 40217806 Free PMC article. Review.
-
Management of complications after valvular interventions.EuroIntervention. 2025 Apr 21;21(8):e390-e410. doi: 10.4244/EIJ-D-24-00066. EuroIntervention. 2025. PMID: 40259838 Review.
-
Transcatheter aortic valve replacement: Past, present, and future.Clin Cardiol. 2024 Jan;47(1):e24209. doi: 10.1002/clc.24209. Clin Cardiol. 2024. PMID: 38269636 Free PMC article. Review.
-
Outcomes of stroke events during transcatheter aortic valve implantation.EuroIntervention. 2022 Jul 22;18(4):e335-e344. doi: 10.4244/EIJ-D-21-00951. EuroIntervention. 2022. PMID: 35135749 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

