Task-related fMRI BOLD response to hyperinsulinemia in healthy older adults
- PMID: 31211691
- PMCID: PMC6675560
- DOI: 10.1172/jci.insight.129700
Task-related fMRI BOLD response to hyperinsulinemia in healthy older adults
Abstract
Background: There is growing evidence to suggest that the brain is an important target for insulin action, and that states of insulin resistance may extend to the CNS with detrimental effects on cognitive functioning. Although the effect of systemic insulin resistance on peripheral organs is well-studied, the degree to which insulin impacts brain function in vivo remains unclear.
Methods: This randomized, single-blinded, 2-way-crossover, sham-controlled, pilot study determined the effects of hyperinsulinemia on fMRI brain activation during a 2-back working memory task in 9 healthy older adults (aged 57-79 years). Each participant underwent two clamp procedures (an insulin infusion and a saline placebo infusion, with normoglycemia maintained during both conditions), to examine the effects of hyperinsulinemia on task performance and associated blood-oxygen-level dependent (BOLD) signal using fMRI.
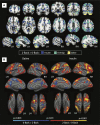
Results: Hyperinsulinemia (compared to saline control) was associated with an increase in both the spatial extent and relative strength of task-related BOLD signal during the 2-back task. Further, the degree of increased task-related activation in select brain regions correlated with greater systemic insulin sensitivity, as well as decreased reaction times and performance accuracy between experimental conditions.
Conclusion: Together, these findings provide evidence of insulin action in the CNS among older adults during periods of sustained cognitive demand, with the greatest effects noted for individuals with highest systemic insulin sensitivity.
Funding: This work was funded by the National Institutes of Health (5R21AG051958, 2016).
Keywords: Aging; Endocrinology; Insulin; Insulin signaling; Neuroimaging.
Conflict of interest statement
Figures



References
-
- [No authors listed]. National Diabetes Statistics Report, 2017. Centers for Disease Control Prevention. https://www.cdc.gov/diabetes/data/statistics-report/index.html Update March 6, 2018. Accessed July 11, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

