Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway
- PMID: 31211693
- PMCID: PMC6675585
- DOI: 10.1172/jci.insight.127729
Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway
Abstract
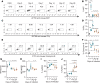
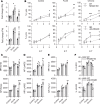
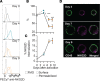
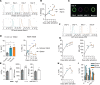
NK cell exhaustion (NCE) due to sustained proliferation results in impaired NK cell function with loss of cytokine production and lytic activity. Using murine models of chronic NK cell stimulation, we have identified a phenotypic signature of NCE characterized by up-regulation of the terminal differentiation marker KLRG1 and by down-regulation of eomesodermin and the activating receptor NKG2D. Chronic stimulation of mice lacking NKG2D resulted in minimized NCE compared to control mice, thus identifying NKG2D as a crucial mediator of NCE. NKG2D internalization and downregulations on NK cells has been previously observed in the presence of tumor cells with high expression of NKG2D ligands (NKG2DL) due to the activation of the DNA damage repair pathways. Interestingly, our study revealed that during NK cell activation there is an increase of MULT1, and NKG2DL, that correlates with an induction of DNA damage. Treatment with the ATM DNA damage repair pathway inhibitor KU55933 (KU) during activation reduced NCE by improving expression of activation markers and genes involved in cell survival, by sustaining NKG2D expression and by preserving cell functionality. Importantly, NK cells expanded ex vivo in the presence of KU displayed increased anti-tumor efficacy in both NKG2D-dependent and -independent mouse models. Collectively, these data demonstrate that NCE is caused by DNA damage and regulated, at least in part, by NKG2D. Further, the prevention of NCE is a promising strategy to improve NK cell-based immunotherapy.
Keywords: DNA repair; Immunology; Immunotherapy; NK cells; Oncology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

