Infection-induced endothelial amyloids impair memory
- PMID: 31211919
- PMCID: PMC6704457
- DOI: 10.1096/fj.201900322R
Infection-induced endothelial amyloids impair memory
Abstract
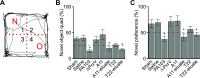
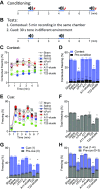
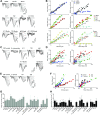
Patients with nosocomial pneumonia exhibit elevated levels of neurotoxic amyloid and tau proteins in the cerebrospinal fluid (CSF). In vitro studies indicate that pulmonary endothelium infected with clinical isolates of either Pseudomonas aeruginosa, Klebsiella pneumoniae, or Staphylococcus aureus produces and releases cytotoxic amyloid and tau proteins. However, the effects of the pulmonary endothelium-derived amyloid and tau proteins on brain function have not been elucidated. Here, we show that P. aeruginosa infection elicits accumulation of detergent insoluble tau protein in the mouse brain and inhibits synaptic plasticity. Mice receiving endothelium-derived amyloid and tau proteins via intracerebroventricular injection exhibit a learning and memory deficit in object recognition, fear conditioning, and Morris water maze studies. We compared endothelial supernatants obtained after the endothelia were infected with P. aeruginosa possessing an intact [P. aeruginosa isolated from patient 103 (PA103) supernatant] or defective [mutant strain of P. aeruginosa lacking a functional type 3 secretion system needle tip complex (ΔPcrV) supernatant] type 3 secretion system. Whereas the PA103 supernatant impaired working memory, the ΔPcrV supernatant had no effect. Immunodepleting amyloid or tau proteins from the PA103 supernatant with the A11 or T22 antibodies, respectively, overtly rescued working memory. Recordings from hippocampal slices treated with endothelial supernatants or CSF from patients with or without nosocomial pneumonia indicated that endothelium-derived neurotoxins disrupted the postsynaptic synaptic response. Taken together, these results establish a plausible mechanism for the neurologic sequelae consequent to nosocomial bacterial pneumonia.-Balczon, R., Pittet, J.-F., Wagener, B. M., Moser, S. A., Voth, S., Vorhees, C. V., Williams, M. T., Bridges, J. P., Alvarez, D. F., Koloteva, A., Xu, Y., Zha, X.-M., Audia, J. P., Stevens, T., Lin, M. T. Infection-induced endothelial amyloids impair memory.
Keywords: cerebrospinal fluid; dementia; depression; learning; nosocomial pneumonia.
Conflict of interest statement
The authors thank the members of the Department of Comparative Medicine and the University of South Alabama for their efforts in animal husbandry, and the members of the Intensive Care Unit team and Clinical Microbiology Laboratory at the University of Alabama at Birmingham for their care in handling and processing clinical samples. The authors also thank Dr. James Maylie (Oregon Health and Science University) for generous help with Igor Pro. This work was funded by a College of Medicine Intramural Award and U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL140182 (to M.T.L.), HL118334 (to J.P.A. and D.F.A.), HL66299 and HL135003 (to T.S. and R.B.), HL60024 (to T.S.), and NIH National Institute of Neurological Disorders and Stroke Grant NS102495 (to X.-M.Z.). S.V. is a predoctoral fellow (T32 HL076125). The authors declare no conflicts of interest.
Figures




References
-
- Patel M. B., Morandi A., Pandharipande P. P. (2015) What’s new in post-ICU cognitive impairment Intensive Care Med. 41, 708–711 - PubMed
-
- Needham D. M., Dinglas V. D., Morris P. E., Jackson J. C., Hough C. L., Mendez-Tellez P. A., Wozniak A. W., Colantuoni E., Ely E. W., Rice T. W., Hopkins R. O., NIH NHLBI ARDS Network (2013) Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am. J. Respir. Crit. Care Med. 188, 567–576 - PMC - PubMed
-
- Annane D., Sharshar T. (2015) Cognitive decline after sepsis. Lancet Respir. Med. 3, 61–69 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

