MicroRNA inhibition upregulates hippocampal A-type potassium current and reduces seizure frequency in a mouse model of epilepsy
- PMID: 31212067
- PMCID: PMC6689429
- DOI: 10.1016/j.nbd.2019.104508
MicroRNA inhibition upregulates hippocampal A-type potassium current and reduces seizure frequency in a mouse model of epilepsy
Abstract
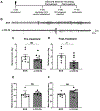
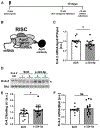
Epilepsy is often associated with altered expression or function of ion channels. One example of such a channelopathy is the reduction of A-type potassium currents in the hippocampal CA1 region. The underlying mechanisms of reduced A-type channel function in epilepsy are unclear. Here, we show that inhibiting a single microRNA, miR-324-5p, which targets the pore-forming A-type potassium channel subunit Kv4.2, selectively increased A-type potassium currents in hippocampal CA1 pyramidal neurons in mice. Resting membrane potential, input resistance and other potassium currents were not altered. In a mouse model of acquired chronic epilepsy, inhibition of miR-324-5p reduced the frequency of spontaneous seizures and interictal epileptiform spikes supporting the physiological relevance of miR-324-5p-mediated control of A-type currents in regulating neuronal excitability. Mechanistic analyses demonstrated that microRNA-induced silencing of Kv4.2 mRNA is increased in epileptic mice leading to reduced Kv4.2 protein levels, which is mitigated by miR-324-5p inhibition. By contrast, other targets of miR-324-5p were unchanged. These results suggest a selective miR-324-5p-dependent mechanism in epilepsy regulating potassium channel function, hyperexcitability and seizures.
Keywords: A-type potassium currents; Antagomir; Epilepsy; Epileptiform spikes; Kv4.2; RISC; RNA-induced silencing complex; Seizures; miR-324-5p; microRNA.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest
C.G. is co-inventor on US patent 9,932,585 B2. All other authors declare no competing financial interests.
Figures


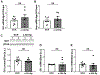

Similar articles
-
MicroRNA-Mediated Downregulation of the Potassium Channel Kv4.2 Contributes to Seizure Onset.Cell Rep. 2016 Sep 27;17(1):37-45. doi: 10.1016/j.celrep.2016.08.074. Cell Rep. 2016. PMID: 27681419 Free PMC article.
-
Estradiol- and Progesterone-Associated Changes in microRNA-Induced Silencing and Reduced Antiseizure Efficacy of an Antagomir in Female Mice.eNeuro. 2023 Jul 24;10(7):ENEURO.0047-22.2023. doi: 10.1523/ENEURO.0047-22.2023. Print 2023 Jul. eNeuro. 2023. PMID: 37433683 Free PMC article.
-
Saikosaponin A modulates remodeling of Kv4.2-mediated A-type voltage-gated potassium currents in rat chronic temporal lobe epilepsy.Drug Des Devel Ther. 2018 Sep 11;12:2945-2958. doi: 10.2147/DDDT.S166408. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30254424 Free PMC article.
-
Epilepsy and microRNA.Neuroscience. 2013 May 15;238:218-29. doi: 10.1016/j.neuroscience.2013.02.027. Epub 2013 Feb 26. Neuroscience. 2013. PMID: 23485811 Review.
-
MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets.Neural Regen Res. 2023 Oct;18(10):2108-2118. doi: 10.4103/1673-5374.369093. Neural Regen Res. 2023. PMID: 37056117 Free PMC article. Review.
Cited by
-
The mechanism of sudden unexpected death in epilepsy: A mini review.Front Neurol. 2023 Feb 6;14:1137182. doi: 10.3389/fneur.2023.1137182. eCollection 2023. Front Neurol. 2023. PMID: 36815002 Free PMC article. Review.
-
The potassium channel Kv4.2 regulates dendritic spine morphology, electroencephalographic characteristics and seizure susceptibility in mice.Exp Neurol. 2020 Dec;334:113437. doi: 10.1016/j.expneurol.2020.113437. Epub 2020 Aug 19. Exp Neurol. 2020. PMID: 32822706 Free PMC article.
-
Identification of key potassium channel genes of temporal lobe epilepsy by bioinformatics analyses and experimental verification.Front Neurol. 2023 Jul 7;14:1175007. doi: 10.3389/fneur.2023.1175007. eCollection 2023. Front Neurol. 2023. PMID: 37483435 Free PMC article.
-
Increased hippocampal excitability in miR-324-null mice.Sci Rep. 2021 May 17;11(1):10452. doi: 10.1038/s41598-021-89874-1. Sci Rep. 2021. PMID: 34001919 Free PMC article.
-
PI3K isoform-selective inhibition in neuron-specific PTEN-deficient mice rescues molecular defects and reduces epilepsy-associated phenotypes.Neurobiol Dis. 2020 Oct;144:105026. doi: 10.1016/j.nbd.2020.105026. Epub 2020 Jul 24. Neurobiol Dis. 2020. PMID: 32712265 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

