Antibacterial-Agent-Immobilized Gelatin Hydrogel as a 3D Scaffold for Natural and Bioengineered Tissues
- PMID: 31212711
- PMCID: PMC6630779
- DOI: 10.3390/gels5020032
Antibacterial-Agent-Immobilized Gelatin Hydrogel as a 3D Scaffold for Natural and Bioengineered Tissues
Abstract
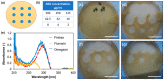
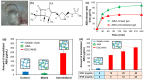
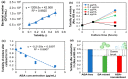
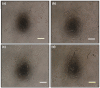
Hydrogels and their medical applications in tissue engineering have been widely studied due to their three-dimensional network structure, biocompatibility, and cell adhesion. However, the development of an artificial bile duct to replace the recipient's tissue is still desired. Some challenges remain in the tissue engineering field, such as infection due to residual artifacts. In other words, at present, there are no established technologies for bile duct reconstruction as strength and biocompatibility problems. Therefore, this study investigated hydrogel as an artificial bile duct base material that can replace tissue without any risk of infectious diseases. First, an antibacterial agent (ABA), Finibax (an ABA used for the clinical treatment of biliary tract infection), was immobilized in gelatin using a crosslinking agent, and the antibacterial properties of the gel and its sustainability were tested. Furthermore, the immobilized amount and the improvement of the proliferation of the human umbilical vein endothelial cells (HUVECs) were cultured as the ABA-Gelatin hydrogel was introduced to prepare a 3D scaffold. Finally, we performed hematoxylin and eosin (H&E) staining after subcutaneous implantation in the rat. Overall, the ABA-Gelatin hydrogel was found to be viable for use in hydrogel applications for tissue engineering due to its good bactericidal ability, cell adhesion, and proliferation, as well as having no cytotoxicity to cells.
Keywords: hydrogel, antibacterial agent, gelatin, 3D scaffold, biomaterials for tissue engineering, cytotoxicity, cell culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
[Gelatin/alginate hydrogel scaffolds prepared by 3D bioprinting promotes cell adhesion and proliferation of human dental pulp cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2017 May 20;37(5):668-672. doi: 10.3969/j.issn.1673-4254.2017.05.17. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 28539292 Free PMC article. Chinese.
-
Effects of 3-dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells.J Endod. 2019 Jun;45(6):706-715. doi: 10.1016/j.joen.2019.03.004. Epub 2019 May 2. J Endod. 2019. PMID: 31056297
-
Co-culture of human umbilical vein endothelial cells and human bone marrow stromal cells into a micro-cavitary gelatin-methacrylate hydrogel system to enhance angiogenesis.Mater Sci Eng C Mater Biol Appl. 2019 Sep;102:906-916. doi: 10.1016/j.msec.2019.04.089. Epub 2019 Apr 29. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31147062
-
Bile Duct Regeneration with an Artificial Bile Duct Made of Gelatin Hydrogel Nonwoven Fabrics.Tissue Eng Part A. 2022 Sep;28(17-18):737-748. doi: 10.1089/ten.TEA.2021.0209. Epub 2022 Jun 21. Tissue Eng Part A. 2022. PMID: 35383474
-
Biocompatibility of hydrogel-based scaffolds for tissue engineering applications.Biotechnol Adv. 2017 Sep;35(5):530-544. doi: 10.1016/j.biotechadv.2017.05.006. Epub 2017 May 27. Biotechnol Adv. 2017. PMID: 28558979 Review.
Cited by
-
Hydrogels for Antitumor and Antibacterial Therapy.Gels. 2022 May 19;8(5):315. doi: 10.3390/gels8050315. Gels. 2022. PMID: 35621613 Free PMC article. Review.
-
Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review.Polymers (Basel). 2021 Jul 14;13(14):2319. doi: 10.3390/polym13142319. Polymers (Basel). 2021. PMID: 34301076 Free PMC article. Review.
-
Therapeutic functions of medical implants from various material categories with integrated biomacromolecular systems.Front Bioeng Biotechnol. 2025 Jan 10;12:1509397. doi: 10.3389/fbioe.2024.1509397. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39867472 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

