Marjoram Relaxes Rat Thoracic Aorta Via a PI3-K/eNOS/cGMP Pathway
- PMID: 31212721
- PMCID: PMC6627793
- DOI: 10.3390/biom9060227
Marjoram Relaxes Rat Thoracic Aorta Via a PI3-K/eNOS/cGMP Pathway
Abstract
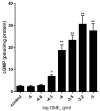
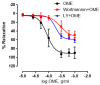
Despite pharmacotherapeutic advances, cardiovascular disease (CVD) remains the primary cause of global mortality. Alternative approaches, such as herbal medicine, continue to be sought to reduce this burden. Origanum majorana is recognized for many medicinal values, yet its vasculoprotective effects remain poorly investigated. Here, we subjected rat thoracic aortae to increasing doses of an ethanolic extract of Origanummajorana (OME). OME induced relaxation in a dose-dependent manner in endothelium-intact rings. This relaxation was significantly blunted in denuded rings. N(ω)-nitro-l-arginine methyl ester (L-NAME) or 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ) significantly reduced the OME-induced vasorelaxation. Cyclic guanosine monophosphate (cGMP) levels were also increased by OME. Moreover, wortmannin or LY294002 significantly reduced OME-induced vasorelaxation. Blockers of ATP-sensitive or Ca2+-activated potassium channels such as glibenclamide or tetraethylamonium (TEA), respectively, did not significantly affect OME-induced relaxation. Similarly, verapamil, a Ca2+ channel blocker, indomethacin, a non-selective cyclooxygenase inhibitor, and pyrilamine, a H1 histamine receptor blocker, did not significantly modulate the observed relaxation. Taken together, our results show that OME induces vasorelaxation via an endothelium-dependent mechanism involving the phosphoinositide 3-kinase (PI3-K)/ endothelial nitric oxide (NO) synthase (eNOS)/cGMP pathway. Our findings further support the medicinal value of marjoram and provide a basis for its beneficial intake. Although consuming marjoram may have an antihypertensive effect, further studies are needed to better determine its effects in different vascular beds.
Keywords: PI3-K; cGMP; hypertension; marjoram; nitric oxide; vasorelaxation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- WHO Hypertension. [(accessed on 22 May 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension.
-
- WHO WHO Traditional Medicine Strategy. [(accessed on 22 May 2019)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

