WC-1 and the Proximal GATA Sequence Mediate a Cis-/Trans-Acting Repressive Regulation of Light-Dependent Gene Transcription in the Dark
- PMID: 31212732
- PMCID: PMC6628569
- DOI: 10.3390/ijms20122854
WC-1 and the Proximal GATA Sequence Mediate a Cis-/Trans-Acting Repressive Regulation of Light-Dependent Gene Transcription in the Dark
Abstract
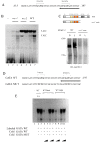
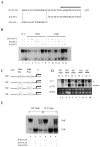
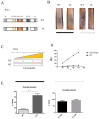
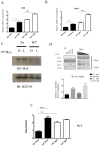
Light influences a wide range of physiological processes from prokaryotes to mammals. Neurospora crassa represents an important model system used for studying this signal pathway. At molecular levels, the WHITE COLLAR Complex (WCC), a heterodimer formed by WC-1 (the blue light photo-sensor) and WC-2 (the transcriptional activator), is the critical positive regulator of light-dependent gene expression. GATN (N indicates any other nucleotide) repeats are consensus sequences within the promoters of light-dependent genes recognized by the WCC. The distal GATN is also known as C-box since it is involved in the circadian clock. However, we know very little about the role of the proximal GATN, and the molecular mechanism that controls the transcription of light-induced genes during the dark/light transition it is still unclear. Here we showed a first indication that mutagenesis of the proximal GATA sequence within the target promoter of the albino-3 gene or deletion of the WC-1 zinc finger domain led to a rise in expression of light-dependent genes already in the dark, effectively decoupling light stimuli and transcriptional activation. This is the first observation of cis-/trans-acting repressive machinery, which is not consistent with the light-dependent regulatory mechanism observed in the eukaryotic world so far.
Keywords: GATA; WC-1; light responses; zinc finger.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

