Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective
- PMID: 31212791
- PMCID: PMC6617096
- DOI: 10.3390/jof5020048
Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective
Abstract
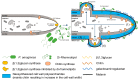
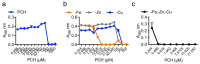
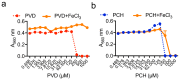
Aspergillus fumigatus and Pseudomonas aeruginosa are central fungal and bacterial members of the pulmonary microbiota. The interactions between A. fumigatus and P. aeruginosa have only just begun to be explored. A balance between inhibitory and stimulatory effects on fungal growth was observed in mixed A. fumigatus-P. aeruginosa cultures. Negative interactions have been seen for homoserine-lactones, pyoverdine and pyochelin resulting from iron starvation and intracellular inhibitory reactive oxidant production. In contrast, several types of positive interactions were recognized. Dirhamnolipids resulted in the production of a thick fungal cell wall, allowing the fungus to resist stress. Phenazines and pyochelin favor iron uptake for the fungus. A. fumigatus is able to use bacterial volatiles to promote its growth. The immune response is also differentially regulated by co-infections.
Keywords: Aspergillus; Pseudomonas; cell wall; cystic fibrosis; interaction; microbiota; phenazine; pyochelin; rhamnolipid; volatile.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Bacterial Interactions with Aspergillus fumigatus in the Immunocompromised Lung.Microorganisms. 2021 Feb 19;9(2):435. doi: 10.3390/microorganisms9020435. Microorganisms. 2021. PMID: 33669831 Free PMC article. Review.
-
Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines.Sci Rep. 2015 Feb 10;5:8220. doi: 10.1038/srep08220. Sci Rep. 2015. PMID: 25665925 Free PMC article.
-
Secondary Metabolites Produced during Aspergillus fumigatus and Pseudomonas aeruginosa Biofilm Formation.mBio. 2022 Aug 30;13(4):e0185022. doi: 10.1128/mbio.01850-22. Epub 2022 Jul 20. mBio. 2022. PMID: 35856657 Free PMC article.
-
Pseudomonas aeruginosa Virulence Factors Support Voriconazole Effects on Aspergillus fumigatus.Pathogens. 2021 Apr 26;10(5):519. doi: 10.3390/pathogens10050519. Pathogens. 2021. PMID: 33925818 Free PMC article.
-
Aspergillus-Pseudomonas interaction, relevant to competition in airways.Med Mycol. 2019 Apr 1;57(Supplement_2):S228-S232. doi: 10.1093/mmy/myy087. Med Mycol. 2019. PMID: 30816973 Review.
Cited by
-
Trends of pulmonary fungal infections from 2013 to 2019: an AI-based real-world observational study in Guangzhou, China.Emerg Microbes Infect. 2021 Dec;10(1):450-460. doi: 10.1080/22221751.2021.1894902. Emerg Microbes Infect. 2021. PMID: 33620282 Free PMC article.
-
Bacterial Interactions with Aspergillus fumigatus in the Immunocompromised Lung.Microorganisms. 2021 Feb 19;9(2):435. doi: 10.3390/microorganisms9020435. Microorganisms. 2021. PMID: 33669831 Free PMC article. Review.
-
How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus.Front Microbiol. 2021 Mar 3;12:617784. doi: 10.3389/fmicb.2021.617784. eCollection 2021. Front Microbiol. 2021. PMID: 33746915 Free PMC article. Review.
-
Live imaging and quantitative analysis of Aspergillus fumigatus growth and morphology during inter-microbial interaction with Pseudomonas aeruginosa.Virulence. 2020 Dec;11(1):1329-1336. doi: 10.1080/21505594.2020.1827885. Virulence. 2020. PMID: 33017225 Free PMC article.
-
Bacterial-fungal interactions and their impact on microbial pathogenesis.Mol Ecol. 2023 May;32(10):2565-2581. doi: 10.1111/mec.16411. Epub 2022 Mar 14. Mol Ecol. 2023. PMID: 35231147 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

