Medical Cannabis Certification in a Large Pediatric Oncology Center
- PMID: 31212902
- PMCID: PMC6617193
- DOI: 10.3390/children6060079
Medical Cannabis Certification in a Large Pediatric Oncology Center
Abstract
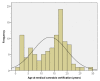
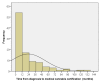
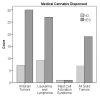
In Minnesota, medical cannabis was approved for use in 2014. From July 2015 to February 2019, our center certified 103 pediatric and young adult patients for the use of medical cannabis under the qualifying conditions of cancer and treatment-related symptoms. Here, we provide a review of the literature on medical cannabis use in pediatric and young adult cancer patients. We also provide demographic data on our patients certified for medical cannabis. The most common diagnoses were leukemia/lymphoma (36%), brain tumors (37%), and malignant solid tumors (26%). The most common indications were chemotherapy-related nausea, pain, and cancer cachexia. The age range at certification was 1.4-28.7 years (median 15.3 years). The time from cancer diagnosis to certification ranged from 0.5-197 months (median 8.9 months). The majority (94%) were certified during their first line of treatment. In the 32 patients who died from recurrent or progressive cancer, the time from certification to death was 1.3-30.3 months (median 4.4 years). Despite requesting certification, a subset (24%) never had medical cannabis dispensed. In our experience, pediatric and young adult oncology patients are interested in medical cannabis to help manage treatment-related symptoms. Ongoing analysis of this data will identify the therapeutic efficacy of medical cannabis.
Keywords: cancer pain; chemotherapy-induced nausea; end-of-life care; medical cannabis; medical marijuana; pediatric oncology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Medical marijuana certification for patients with sickle cell disease: a report of a single center experience.Blood Adv. 2020 Aug 25;4(16):3814-3821. doi: 10.1182/bloodadvances.2020002325. Blood Adv. 2020. PMID: 32790846 Free PMC article.
-
Pediatric Patient Experiences Using Medical Cannabis in Cancer Symptom Management as Reported by Parents of Children and Adolescents and by Young Adults.J Pediatr Hematol Oncol Nurs. 2025 Jan-Feb;42(1-2):37-43. doi: 10.1177/27527530251318606. Epub 2025 May 29. J Pediatr Hematol Oncol Nurs. 2025. PMID: 40438918
-
Integrated Medical Cannabis Consultations in a Palliative Care Program: Policies, Procedures, and Progress after Six Years of Practice.J Palliat Med. 2022 May;25(5):802-806. doi: 10.1089/jpm.2021.0577. Epub 2022 Mar 18. J Palliat Med. 2022. PMID: 35319304
-
Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs.Res Social Adm Pharm. 2016 Jul-Aug;12(4):638-54. doi: 10.1016/j.sapharm.2015.09.002. Epub 2015 Sep 16. Res Social Adm Pharm. 2016. PMID: 26443472 Review.
-
Cannabis for cancer - illusion or the tip of an iceberg: a review of the evidence for the use of Cannabis and synthetic cannabinoids in oncology.Expert Opin Investig Drugs. 2019 Mar;28(3):285-296. doi: 10.1080/13543784.2019.1561859. Epub 2018 Dec 29. Expert Opin Investig Drugs. 2019. PMID: 30572744 Review.
Cited by
-
The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology.Cancers (Basel). 2021 Jan 5;13(1):157. doi: 10.3390/cancers13010157. Cancers (Basel). 2021. PMID: 33466435 Free PMC article. Review.
-
Cannabinoids for Medical Purposes in Children: A Living Systematic Review.Acta Paediatr. 2025 Sep;114(9):2148-2159. doi: 10.1111/apa.70140. Epub 2025 May 28. Acta Paediatr. 2025. PMID: 40437694 Free PMC article. Review.
-
Efficacy and safety of medical cannabinoids in children: a systematic review and meta-analysis.Sci Rep. 2021 Dec 6;11(1):23462. doi: 10.1038/s41598-021-02770-6. Sci Rep. 2021. PMID: 34873203 Free PMC article.
-
Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma.Cancers (Basel). 2021 Jan 18;13(2):330. doi: 10.3390/cancers13020330. Cancers (Basel). 2021. PMID: 33477420 Free PMC article.
-
Perspectives of pediatric oncologists and palliative care physicians on the therapeutic use of cannabis in children with cancer.Cancer Rep (Hoboken). 2022 Sep;5(9):e1551. doi: 10.1002/cnr2.1551. Epub 2021 Oct 21. Cancer Rep (Hoboken). 2022. PMID: 34672127 Free PMC article.
References
-
- Minnesota Department of Health: Medical Cannabis. [(accessed on 31 January 2019)]; Available online: https://www.health.state.mn.us/people/cannabis/index.html.
-
- Minnesota Department of Health Medical Cannabis Program Update. [(accessed on 31 January 2019)]; Available online: https://www.health.state.mn.us/people/cannabis/docs/about/update0119.pdf.
LinkOut - more resources
Full Text Sources

