The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals
- PMID: 31212992
- PMCID: PMC6627200
- DOI: 10.3390/nu11061363
The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals
Abstract
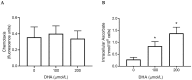
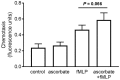
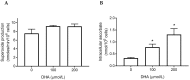
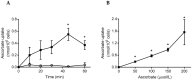
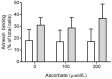
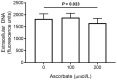
Vitamin C (ascorbate) is important for neutrophil function and immune health. Studies showing improved immune function have primarily used cells from scorbutic animals or from individuals with infectious conditions or immune cell disorders. Few studies have focused on the requirements of neutrophils from healthy adults. Therefore, we have investigated the role of vitamin C, at concentrations equivalent to those obtained in plasma from oral intakes (i.e., 50-200 µmol/L), on key functions of neutrophils isolated from healthy individuals. Cells were either pre-loaded with dehydroascorbic acid, which is rapidly reduced intracellularly to ascorbate, or the cells were activated in the presence of extracellular ascorbate. We measured the effects of enhanced ascorbate uptake on the essential functions of chemotaxis, oxidant production, programmed cell death and neutrophil extracellular trap (NET) formation. We found that neutrophils isolated from healthy individuals already had replete ascorbate status (0.35 nmol/106 cells), therefore they did not uptake additional ascorbate. However, they readily took up dehydroascorbic acid, thus significantly increasing their intracellular ascorbate concentrations, although this was found to have no additional effect on superoxide production or chemotaxis. Interestingly, extracellular ascorbate appeared to enhance directional mobilityin the presence of the chemoattractant formyl-methionyl-leucyl-phenylalanine (fMLP). Stimulation of the cells in the presence of ascorbate significantly increased intracellular ascorbate concentrations and, although this exhibited a non-significant increase in phosphatidylserine exposure, NET formation was significantly attenuated. Our findings demonstrate the ability of neutrophils to regulate their uptake of ascorbate from the plasma of healthy humans to maintain an optimal level within the cell for proper functioning. Higher oral intakes, however, may help reduce tissue damage and inflammatory pathologies associated with NET formation.
Keywords: ascorbate; chemotaxis; immunity; neutrophil extracellular traps; neutrophils; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

