Simple mechanics of protein machines
- PMID: 31213170
- PMCID: PMC6597773
- DOI: 10.1098/rsif.2019.0244
Simple mechanics of protein machines
Abstract
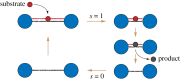
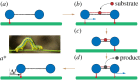
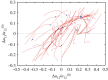
While belonging to the nanoscale, protein machines are so complex that tracing even a small fraction of their cycle requires weeks of calculations on supercomputers. Surprisingly, many aspects of their operation can be however already reproduced by using very simple mechanical models of elastic networks. The analysis suggests that, similar to other self-organized complex systems, functional collective dynamics in such proteins is effectively reduced to a low-dimensional attractive manifold.
Keywords: allosteric effects; complex systems; elastic networks; mechanochemical motions in enzymes; molecular motors; self-organization.
Conflict of interest statement
We declare we have no competing interests.
Figures



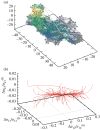

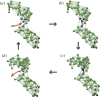
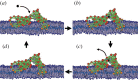
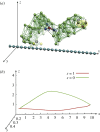

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
