Individualised screening for diabetic retinopathy: the ISDR study-rationale, design and methodology for a randomised controlled trial comparing annual and individualised risk-based variable-interval screening
- PMID: 31213445
- PMCID: PMC6588999
- DOI: 10.1136/bmjopen-2018-025788
Individualised screening for diabetic retinopathy: the ISDR study-rationale, design and methodology for a randomised controlled trial comparing annual and individualised risk-based variable-interval screening
Abstract
Introduction: Currently, all people with diabetes (PWD) aged 12 years and over in the UK are invited for screening for diabetic retinopathy (DR) annually. Resources are not increasing despite a 5% increase in the numbers of PWD nationwide each year. We describe the rationale, design and methodology for a randomised controlled trial (RCT) evaluating the safety, acceptability and cost-effectiveness of personalised variable-interval risk-based screening for DR. This is the first randomised trial of personalised screening for DR and the largest ophthalmic RCT in the UK.
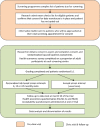
Methods and analysis: PWD attending seven screening clinics in the Liverpool Diabetic Eye Screening Programme were recruited into a single site RCT with a 1:1 allocation to individualised risk-based variable-interval or annual screening intervals. A risk calculation engine developed for the trial estimates the probability that an individual will develop referable disease (screen positive DR) within the next 6, 12 or 24 months using demographic, retinopathy and systemic risk factor data from primary care and screening programme records. Dynamic, secure, real-time data connections have been developed. The primary outcome is attendance for follow-up screening. We will test for equivalence in attendance rates between the two arms. Secondary outcomes are rates and severity of DR, visual outcomes, cost-effectiveness and health-related quality of life. The required sample size was 4460 PWD. Recruitment is complete, and the trial is in follow-up.
Ethics and dissemination: Ethical approval was obtained from National Research Ethics Service Committee North West - Preston, reference 14/NW/0034. Results will be presented at international meetings and published in peer-reviewed journals. This pragmatic RCT will inform screening policy in the UK and elsewhere.
Trial registration number: ISRCTN87561257; Pre-results.
Keywords: diabetic retinopathy; general diabetes; health economics; health policy; medical ophthalmology; medical retina.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- PHE bulletin. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil... (Last accessed 03 Jan 2018).
-
- Klein R, Klein BE, Moss SE, et al. . The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520–6. - PubMed
-
- Klein R, Klein BE, Moss SE, et al. . The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527–32. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
