Morphological and functional correlates of vestibular synaptic deafferentation and repair in a mouse model of acute-onset vertigo
- PMID: 31213478
- PMCID: PMC6679379
- DOI: 10.1242/dmm.039115
Morphological and functional correlates of vestibular synaptic deafferentation and repair in a mouse model of acute-onset vertigo
Abstract
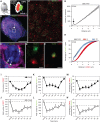
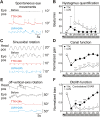
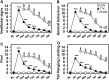
Damage to cochlear primary afferent synapses has been shown to be a key factor in various auditory pathologies. Similarly, the selective lesioning of primary vestibular synapses might be an underlying cause of peripheral vestibulopathies that cause vertigo and dizziness, for which the pathophysiology is currently unknown. To thoroughly address this possibility, we selectively damaged the synaptic contacts between hair cells and primary vestibular neurons in mice through the transtympanic administration of a glutamate receptor agonist. Using a combination of histological and functional approaches, we demonstrated four key findings: (1) selective synaptic deafferentation is sufficient to generate acute vestibular syndrome with characteristics similar to those reported in patients; (2) the reduction of the vestibulo-ocular reflex and posturo-locomotor deficits mainly depends on spared synapses; (3) damaged primary vestibular synapses can be repaired over the days and weeks following deafferentation; and (4) the synaptic repair process occurs through the re-expression and re-pairing of synaptic proteins such as CtBP2 and SHANK-1. Primary synapse repair might contribute to re-establishing the initial sensory network. Deciphering the molecular mechanism that supports synaptic repair could offer a therapeutic opportunity to rescue full vestibular input and restore gait and balance in patients.
Keywords: Excitotoxicity; Plasticity; Synapses; Vestibular disorders; Vestibule.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: a rat model of vertigo symptoms.Dis Model Mech. 2016 Oct 1;9(10):1181-1192. doi: 10.1242/dmm.024521. Epub 2016 Jun 29. Dis Model Mech. 2016. PMID: 27483344 Free PMC article.
-
Peripheral vestibular plasticity vs central compensation: evidence and questions.J Neurol. 2019 Sep;266(Suppl 1):27-32. doi: 10.1007/s00415-019-09388-9. Epub 2019 May 27. J Neurol. 2019. PMID: 31134376 Review.
-
Reduced Balance Restoration Capacities Following Unilateral Vestibular Insult in Elderly Mice.Front Neurol. 2018 Jun 25;9:462. doi: 10.3389/fneur.2018.00462. eCollection 2018. Front Neurol. 2018. PMID: 29988508 Free PMC article.
-
Vertigo and balance in children--diagnostic approach and insights from imaging.Eur J Paediatr Neurol. 2011 Jul;15(4):289-94. doi: 10.1016/j.ejpn.2011.04.010. Epub 2011 May 14. Eur J Paediatr Neurol. 2011. PMID: 21571558 Review.
-
Structure and function of the hair cell ribbon synapse.J Membr Biol. 2006 Feb-Mar;209(2-3):153-65. doi: 10.1007/s00232-005-0854-4. Epub 2006 May 25. J Membr Biol. 2006. PMID: 16773499 Free PMC article. Review.
Cited by
-
Assessment of cochlear toxicity in response to chronic 3,3'-iminodipropionitrile in mice reveals early and reversible functional loss that precedes overt histopathology.Arch Toxicol. 2021 Mar;95(3):1003-1021. doi: 10.1007/s00204-020-02962-5. Epub 2021 Jan 25. Arch Toxicol. 2021. PMID: 33495873 Free PMC article.
-
Surgical techniques and functional evaluation for vestibular lesions in the mouse: unilateral labyrinthectomy (UL) and unilateral vestibular neurectomy (UVN).J Neurol. 2020 Dec;267(Suppl 1):51-61. doi: 10.1007/s00415-020-09960-8. Epub 2020 Jun 17. J Neurol. 2020. PMID: 32556569 Free PMC article.
-
L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects.Cells. 2022 Feb 16;11(4):684. doi: 10.3390/cells11040684. Cells. 2022. PMID: 35203333 Free PMC article.
-
Gut Microbiome and Metabolome Changes in Mice With Acute Vestibular Deficit.Front Cell Infect Microbiol. 2022 Apr 4;12:821780. doi: 10.3389/fcimb.2022.821780. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35444956 Free PMC article.
-
Cellular and Molecular Mechanisms of Vestibular Ageing.J Clin Med. 2023 Aug 25;12(17):5519. doi: 10.3390/jcm12175519. J Clin Med. 2023. PMID: 37685587 Free PMC article. Review.
References
-
- Braude J. P., Vijayakumar S., Baumgarner K., Laurine R., Jones T. A., Jones S. M. and Pyott S. J. (2015). Deletion of Shank1 has minimal effects on the molecular composition and function of glutamatergic afferent post synapses in the mouse inner ear. Hear. Res. 321, 52-64. 10.1016/j.heares.2015.01.008 - DOI - PMC - PubMed

