Changing Carrot Color: Insertions in DcMYB7 Alter the Regulation of Anthocyanin Biosynthesis and Modification
- PMID: 31213511
- PMCID: PMC6716245
- DOI: 10.1104/pp.19.00523
Changing Carrot Color: Insertions in DcMYB7 Alter the Regulation of Anthocyanin Biosynthesis and Modification
Abstract
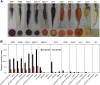
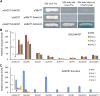
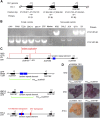
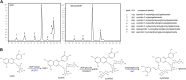
The original domesticated carrots (Daucus carota) are thought to have been purple, accumulating large quantities of anthocyanins in their roots. A quantitative trait locus associated with anthocyanin pigmentation in purple carrot roots has been identified on chromosome 3 and includes two candidate genes, DcMYB6 and DcMYB7 Here, we characterized the functions of DcMYB6 and DcMYB7 in carrots. Overexpression of DcMYB7, but not DcMYB6, in the orange carrot 'Kurodagosun' led to anthocyanin accumulation in roots. Knockout of DcMYB7 in the solid purple (purple periderm, phloem, and xylem) carrot 'Deep Purple' using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 system resulted in carrots with yellow roots. DcMYB7 could activate the expression of its DcbHLH3 partner, a homolog of the anthocyanin-related apple (Malus × domestica) bHLH3, and structural genes in the anthocyanin biosynthetic pathway. We determined that the promoter sequence of DcMYB7 in nonpurple carrots was interrupted either by DcMYB8, a nonfunctional tandem duplication of DcMYB7, or by two transposons, leading to the transcriptional inactivation of DcMYB7 in nonpurple carrot roots. As a result, nonpurple carrots fail to accumulate anthocyanins in their roots. Our study supports the hypothesis that another genetic factor suppresses DcMYB7 expression in the phloem and xylem of purple peridermal carrot root tissues. DcMYB7 also regulated the glycosylation and acylation of anthocyanins by directly activating DcUCGXT1 and DcSAT1 We reveal the genetic factors conditioning anthocyanin pigmentation in purple versus nonpurple carrot roots. Our results also provide insights into the mechanisms underlying anthocyanin glycosylation and acylation.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures


Comment in
-
Purple Is the New Orange: Anthocyanin Regulation Coming Together in Carrot.Plant Physiol. 2019 Sep;181(1):12-13. doi: 10.1104/pp.19.00910. Plant Physiol. 2019. PMID: 31467141 Free PMC article. No abstract available.
References
-
- Arscott SA, Tanumihardjo SA (2010) Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr Rev Food Sci 9: 223–239
-
- Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48: 958–970 - PubMed
-
- Banga O. (1963) Origin and distribution of the western cultivated carrot. Genet Agrar 17: 357–370
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

