PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension
- PMID: 31213542
- PMCID: PMC6613097
- DOI: 10.1073/pnas.1821401116
PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension
Abstract
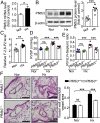
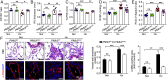
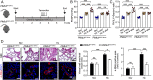
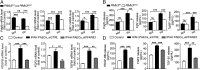
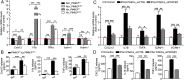
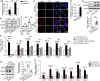
Increased glycolysis in the lung vasculature has been connected to the development of pulmonary hypertension (PH). We therefore investigated whether glycolytic regulator 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (PFKFB3)-mediated endothelial glycolysis plays a critical role in the development of PH. Heterozygous global deficiency of Pfkfb3 protected mice from developing hypoxia-induced PH, and administration of the PFKFB3 inhibitor 3PO almost completely prevented PH in rats treated with Sugen 5416/hypoxia, indicating a causative role of PFKFB3 in the development of PH. Immunostaining of lung sections and Western blot with isolated lung endothelial cells showed a dramatic increase in PFKFB3 expression and activity in pulmonary endothelial cells of rodents and humans with PH. We generated mice that were constitutively or inducibly deficient in endothelial Pfkfb3 and found that these mice were incapable of developing PH or showed slowed PH progression. Compared with control mice, endothelial Pfkfb3-knockout mice exhibited less severity of vascular smooth muscle cell proliferation, endothelial inflammation, and leukocyte recruitment in the lungs. In the absence of PFKFB3, lung endothelial cells from rodents and humans with PH produced lower levels of growth factors (such as PDGFB and FGF2) and proinflammatory factors (such as CXCL12 and IL1β). This is mechanistically linked to decreased levels of HIF2A in lung ECs following PFKFB3 knockdown. Taken together, these results suggest that targeting PFKFB3 is a promising strategy for the treatment of PH.
Keywords: endothelial cells; glycolysis; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tuder R. M., Pathology of pulmonary arterial hypertension. Semin. Respir. Crit. Care Med. 30, 376–385 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

