A Differential Approach to Form and Site of Peptic Ulcer
- PMID: 31213634
- PMCID: PMC6582142
- DOI: 10.1038/s41598-019-44893-x
A Differential Approach to Form and Site of Peptic Ulcer
Abstract
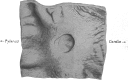
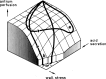
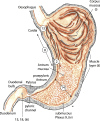
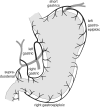
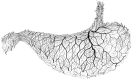
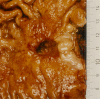
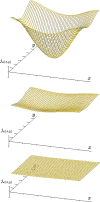
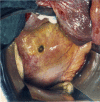
The structural organization of intestinal blood flow is such as to allow for intramural collateral flow. Redistribution phenomena due to different local metabolic demands may lead to an impaired perfusion of parts of the intestinal wall which will display a characteristic pattern. Based on Ohm's and Kirchhoff's laws, a differential analysis of the gastric vascular bed bridges the gap between basic physiological concepts and traditional anatomical, pathological and clinical knowledge. An ulcer of the intestinal wall becomes understandable as a non-occlusive infarct based on a supply/demand conflict in an anisotropic structure as it can be found in the upper and lower gastrointestinal tract of man.
Conflict of interest statement
The authors declare no competing interests.
Figures










Similar articles
-
Gastric hypermotility and antropyloric dysfunction in gastric ulcer patients.Br J Surg. 1982 Jan;69(1):11-13. doi: 10.1002/bjs.1800690105. Br J Surg. 1982. PMID: 7053794
-
[New views on the physiopathology of peptic ulcer].MMW Munch Med Wochenschr. 1974 Feb 1;116(5):181-4. MMW Munch Med Wochenschr. 1974. PMID: 4149847 German. No abstract available.
-
Neuromuscular tissue defects and antropyloric dysfunction in peptic ulcer.Scand J Gastroenterol Suppl. 1981;67:111-3. Scand J Gastroenterol Suppl. 1981. PMID: 6941385
-
[Relationship of pathological changes in the stomach to chronic nonspecific lung diseases].Klin Med (Mosk). 1975 May;52(5):38-43. Klin Med (Mosk). 1975. PMID: 1094181 Review. Russian. No abstract available.
-
ABC of the upper gastrointestinal tract: Pathophysiology of duodenal and gastric ulcer and gastric cancer.BMJ. 2001 Oct 27;323(7319):980-2. doi: 10.1136/bmj.323.7319.980. BMJ. 2001. PMID: 11679389 Free PMC article. Review. No abstract available.
Cited by
-
Risk factors for peptic ulcer bleeding one year after the initial episode.Gut Pathog. 2024 Nov 28;16(1):71. doi: 10.1186/s13099-024-00669-x. Gut Pathog. 2024. PMID: 39609846 Free PMC article.
-
Therapeutic Promises of Medicinal Plants in Bangladesh and Their Bioactive Compounds against Ulcers and Inflammatory Diseases.Plants (Basel). 2021 Jul 1;10(7):1348. doi: 10.3390/plants10071348. Plants (Basel). 2021. PMID: 34371551 Free PMC article. Review.
-
Unusual Complicated Gastric Ulcers.Medicina (Kaunas). 2021 Dec 9;57(12):1345. doi: 10.3390/medicina57121345. Medicina (Kaunas). 2021. PMID: 34946290 Free PMC article. Review.
-
Ulcer Scarring in the Gastric Conduit Is a Risk Factor for Anastomotic Leakage After Esophagectomy for Esophageal Cancer.J Gastrointest Surg. 2023 Feb;27(2):250-261. doi: 10.1007/s11605-022-05545-0. Epub 2022 Dec 12. J Gastrointest Surg. 2023. PMID: 36509899
References
-
- Aschoff L. Ueber die mechanischen Momente in der Pathogenese des runden Magengeschwürs und über seine Beziehungen zum Krebs. DMW-Deutsche Medizinische Wochenschrift. 1912;38:494–496. doi: 10.1055/s-0029-1189360. - DOI
-
- Virchow R. Historisches, Kritisches und Positives zur Lehre der Unterleibsaffektionen. Virchows Archiv. 1853;5:281–375. doi: 10.1007/BF01878692. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

