Treatment patterns and economic outcomes in patients with juvenile idiopathic arthritis
- PMID: 31213863
- PMCID: PMC6549432
- DOI: 10.2147/CEOR.S197117
Treatment patterns and economic outcomes in patients with juvenile idiopathic arthritis
Abstract
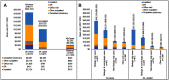
Purpose: To describe health care resource utilization (HCRU) and costs among patients with juvenile idiopathic arthritis (JIA) compared to patients without JIA and to describe treatment patterns among JIA patients who initiated biologic and non-biologic disease-modifying antirheumatic drugs (DMARDs). Patients and methods: The IBM MarketScan® Commercial Database was used to identify patients aged 2-17 years with a new JIA diagnosis (index date) and 12 months continuous enrollment pre- and post-diagnosis from 2008 to 2016. JIA patients were matched to non-JIA patients on age, gender, region, and health plan type. Patients with other rheumatic or autoimmune conditions were excluded. Receipt of a biologic and/or non-biologic was evaluated on or after the new JIA diagnosis. Results: A total of 3,815 JIA patients were matched to 11,535 non-JIA patients (mean age 10.0 [SD=4.5], 69% female). Average total costs were greater for JIA patients than non-JIA controls ($18,611 [SD=$42,104; median=$8,189] versus $2,203 [SD=$9,309; median=$649], p<0.001). Outpatient pharmacy costs were 33.6% of the total costs among JIA patients compared to 18.4% among non-JIA patients (p<0.001). The proportion of inpatient cost (11.4% versus 14.3%, p<0.001) and outpatient costs (55% versus 67.4%, p<0.001) of total costs was lower among JIA patients compared to non-JIA patients. Patients with 12 months of continuous enrollment post-treatment initiation (n=2,014) were classified as non-biologic only (n=734), biologic only (n=873), and both biologic and non-biologic (n=407) users. Among biologic and non-biologic users, 41.1% and 56.8% were persistent on their index medication for 12 months. Of patients treated with a biologic only, TNF inhibitors (TNFi) comprised 87.1% of the total treatment costs. Conclusion: JIA is associated with increased costs and utilization in every HCRU category compared to matched non-JIA patients. While JIA-related costs varied by treatment cohort, patients on biologic DMARDs had substantially higher costs than patients on non-biologic DMARDs and fewer than one-half were persistent at 12 months after biologic initiation.
Keywords: administrative claims; antirheumatic agent; health expenditures; juvenile arthritis; medication adherence.
Conflict of interest statement
Alexander Marshall, Kiran Gupta, and Michael Pazirandeh are employed by Bristol-Myers Squibb. Machaon Bonafede and Donna McMorrow are employed by Truven Health Analytics, an IBM Watson Health business and received funding from Bristol-Myers Squibb to conduct this study. The authors report no other conflicts of interest in this work. This research was presented in part at ISPOR 2018 in Baltimore, MD, USA and at the Annual European Congress of Rheumatology in Amsterdam, Netherlands.
Figures
Similar articles
-
Economic impact of Juvenile Idiopathic Arthritis: a systematic review.Pediatr Rheumatol Online J. 2021 Oct 9;19(1):152. doi: 10.1186/s12969-021-00641-y. Pediatr Rheumatol Online J. 2021. PMID: 34627296 Free PMC article.
-
Use of Tumor Necrosis Factor-Alpha Inhibitors in Children and Young Adults With Juvenile Idiopathic Arthritis or Rheumatoid Arthritis.Pharmacotherapy. 2016 Dec;36(12):1201-1209. doi: 10.1002/phar.1856. Epub 2016 Dec 4. Pharmacotherapy. 2016. PMID: 27779782
-
Treatment patterns and health care costs for patients with psoriatic arthritis on biologic therapy: a retrospective cohort study.Clin Ther. 2013 Sep;35(9):1376-85. doi: 10.1016/j.clinthera.2013.07.328. Epub 2013 Aug 15. Clin Ther. 2013. PMID: 23954091
-
Evaluation of Real-World Healthcare Resource Utilization and Associated Costs in Children with Juvenile Idiopathic Arthritis: A Canadian Retrospective Cohort Study.Rheumatol Ther. 2021 Sep;8(3):1303-1322. doi: 10.1007/s40744-021-00331-x. Epub 2021 Jul 18. Rheumatol Ther. 2021. PMID: 34275124 Free PMC article.
-
Economic Burden and Treatment Patterns of Cycling between Conventional Synthetic Disease-modifying Antirheumatic Drugs Among Biologic-treated Patients with Rheumatoid Arthritis.Clin Ther. 2016 May;38(5):1205-16. doi: 10.1016/j.clinthera.2016.03.013. Epub 2016 Apr 2. Clin Ther. 2016. PMID: 27045991
Cited by
-
Economic impact of Juvenile Idiopathic Arthritis: a systematic review.Pediatr Rheumatol Online J. 2021 Oct 9;19(1):152. doi: 10.1186/s12969-021-00641-y. Pediatr Rheumatol Online J. 2021. PMID: 34627296 Free PMC article.
-
Trends in New Use of Disease-Modifying Antirheumatic Drugs for Juvenile Idiopathic Arthritis Among Commercially Insured Children in the United States from 2001 to 2022.Arthritis Rheumatol. 2025 Apr;77(4):468-476. doi: 10.1002/art.43041. Epub 2024 Dec 10. Arthritis Rheumatol. 2025. PMID: 39435602 Free PMC article.
-
Incidence, prevalence, and comorbidities of juvenile idiopathic arthritis in Germany: a retrospective observational cohort health claims database study.Pediatr Rheumatol Online J. 2022 Nov 16;20(1):100. doi: 10.1186/s12969-022-00755-x. Pediatr Rheumatol Online J. 2022. PMID: 36384690 Free PMC article.
-
Infliximab therapy and outcomes in patients with polyarticular juvenile idiopathic arthritis: a single-center study in China.World J Pediatr. 2020 Feb;16(1):68-73. doi: 10.1007/s12519-019-00316-5. Epub 2019 Oct 14. World J Pediatr. 2020. PMID: 31612428
-
The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases.Int J Mol Sci. 2019 Nov 23;20(23):5875. doi: 10.3390/ijms20235875. Int J Mol Sci. 2019. PMID: 31771129 Free PMC article. Review.
References
-
- Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. - PubMed
-
- Manners PJ, Diepeveen DA. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics. 1996;98:84–90. - PubMed
-
- Beukelman T, Patkar Nivedita M, Saag KG, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;2011(63):465–482. doi:10.1002/acr.20460 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

