Humanin, a Mitochondrial-Derived Peptide Released by Astrocytes, Prevents Synapse Loss in Hippocampal Neurons
- PMID: 31214013
- PMCID: PMC6555273
- DOI: 10.3389/fnagi.2019.00123
Humanin, a Mitochondrial-Derived Peptide Released by Astrocytes, Prevents Synapse Loss in Hippocampal Neurons
Abstract
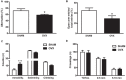
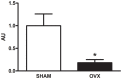
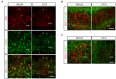
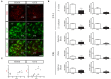
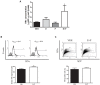
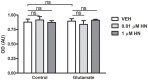
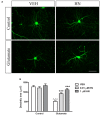
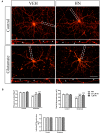
Astroglial cells are crucial for central nervous system (CNS) homeostasis. They undergo complex morpho-functional changes during aging and in response to hormonal milieu. Ovarian hormones positively affect different astroglia parameters, including regulation of cell morphology and release of neurotrophic and neuroprotective factors. Thus, ovarian hormone loss during menopause has profound impact in astroglial pathophysilogy and has been widely associated to the process of brain aging. Humanin (HN) is a secreted mitochondrial-encoded peptide with neuroprotective effects. It is localized in several tissues with high metabolic rate and its expression decreases with age. In the brain, humanin has been found in glial cells in physiological conditions. We previously reported that surgical menopause induces hippocampal mitochondrial dysfunction that mimics an aging phenotype. However, the effect of ovarian hormone deprivation on humanin expression in this area has not been studied. Also, whether astrocytes express and release humanin and the regulation of such processes by ovarian hormones remain elusive. Although humanin has also proven to be beneficial in ameliorating cognitive impairment induced by different insults, its putative actions on structural synaptic plasticity have not been fully addressed. In a model of surgical menopause in rats, we studied hippocampal humanin expression and localization by real-time quantitative polymerase chain reaction (RT-qPCR) and double immunohistochemistry, respectively. Humanin production and release and ovarian hormone regulation of such processes were studied in cultured astrocytes by flow cytometry and ELISA, respectively. Humanin effects on glutamate-induced structural synaptic alterations were determined in primary cultures of hippocampal neurons by immunocytochemistry. Humanin expression was lower in the hippocampus of ovariectomized rats and its immunoreactivity colocalized with astroglial markers. Chronic ovariectomy also promoted the presence of less complex astrocytes in this area. Ovarian hormones increased humanin intracellular content and release by cultured astrocytes. Humanin prevented glutamate-induced dendritic atrophy and reduction in puncta number and total puncta area for pre-synaptic marker synaptophysin in cultured hippocampal neurons. In conclusion, astroglial functional and morphological alterations induced by chronic ovariectomy resemble an aging phenotype and could affect astroglial support to neuronal function by altering synaptic connectivity and functionality. Reduced astroglial-derived humanin may represent an underlying mechanism for synaptic dysfunction and cognitive decline after menopause.
Keywords: astrocytes; hippocampus; humanin; mitochondria; ovarian hormones; synapse.
Figures
Similar articles
-
Cross-talk signals in the CNS: role of neurotrophic and hormonal factors, adhesion molecules and intercellular signaling agents in luteinizing hormone-releasing hormone (LHRH)-astroglial interactive network.Front Biosci. 1997 Mar 1;2:d88-125. doi: 10.2741/a177. Front Biosci. 1997. PMID: 9159216 Review.
-
The Mitochondria-Derived Peptide Humanin Improves Recovery from Intracerebral Hemorrhage: Implication of Mitochondria Transfer and Microglia Phenotype Change.J Neurosci. 2020 Mar 4;40(10):2154-2165. doi: 10.1523/JNEUROSCI.2212-19.2020. Epub 2020 Jan 24. J Neurosci. 2020. PMID: 31980585 Free PMC article.
-
Hormone deprivation alters mitochondrial function and lipid profile in the hippocampus.J Endocrinol. 2017 Apr;233(1):1-14. doi: 10.1530/JOE-16-0451. Epub 2017 Jan 27. J Endocrinol. 2017. PMID: 28130408
-
The LHRH-astroglial network of signals as a model to study neuroimmune interactions: assessment of messenger systems and transduction mechanisms at cellular and molecular levels.Neuroimmunomodulation. 1996 Jan-Feb;3(1):1-27. doi: 10.1159/000097223. Neuroimmunomodulation. 1996. PMID: 8892357 Review.
-
Chronic Unexpected Mild Stress Destroys Synaptic Plasticity of Neurons through a Glutamate Transporter, GLT-1, of Astrocytes in the Ischemic Stroke Rat.Neural Plast. 2019 Mar 25;2019:1615925. doi: 10.1155/2019/1615925. eCollection 2019. Neural Plast. 2019. PMID: 31019528 Free PMC article.
Cited by
-
Hippocampal neurons isolated from rats subjected to the valproic acid model mimic in vivo synaptic pattern: evidence of neuronal priming during early development in autism spectrum disorders.Mol Autism. 2021 Mar 6;12(1):23. doi: 10.1186/s13229-021-00428-8. Mol Autism. 2021. PMID: 33676530 Free PMC article.
-
Transcriptome analyses of the cortex and white matter of focal cortical dysplasia type II: Insights into pathophysiology and tissue characterization.Front Neurol. 2023 Mar 15;14:1023950. doi: 10.3389/fneur.2023.1023950. eCollection 2023. Front Neurol. 2023. PMID: 37006485 Free PMC article.
-
Decoding the rosetta stone of mitonuclear communication.Pharmacol Res. 2020 Nov;161:105161. doi: 10.1016/j.phrs.2020.105161. Epub 2020 Aug 23. Pharmacol Res. 2020. PMID: 32846213 Free PMC article. Review.
-
Neuroprotective astroglial response to neural damage and its relevance to affective disorders.Explor Neuroprotective Ther. 2023;3(5):328-345. doi: 10.37349/ent.2023.00054. Epub 2023 Oct 31. Explor Neuroprotective Ther. 2023. PMID: 37920189 Free PMC article.
-
Hyperbaric Oxygen Treatment-From Mechanisms to Cognitive Improvement.Biomolecules. 2021 Oct 15;11(10):1520. doi: 10.3390/biom11101520. Biomolecules. 2021. PMID: 34680155 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

