Gray Matter Atrophy to Explain Subclinical Oculomotor Deficit in Multiple Sclerosis
- PMID: 31214114
- PMCID: PMC6558169
- DOI: 10.3389/fneur.2019.00589
Gray Matter Atrophy to Explain Subclinical Oculomotor Deficit in Multiple Sclerosis
Abstract
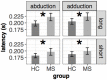
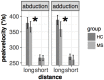
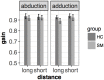
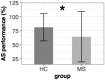
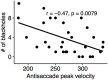
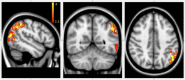
Eye movement deficits are frequently noted in multiple sclerosis during bedside clinical examination, but subtle dysfunction may remain undetected and might only be identified with advanced approaches. While classical neurology provides insight into the complex functional anatomy of oculomotor functions, little is known about the structural background of this dysfunction in MS. Thirty four clinically stable, treated relapsing-remitting MS patients with mild disability and 34 healthy controls were included in our study. Group difference and correlation with clinical parameters were analyzed in case of the latency, peak-velocity, gain, dysconjugacy index, and performance during a saccade and anti-saccade task. High-resolution T1 weighted, T2 FLAIR, and double inversion recovery images were acquired on 3T to evaluate the correlation between behavioral and MRI parameters, such as T2 lesion and T1 black-hole burden, global brain, gray, and white matter atrophy. VBM style analysis was used to identify the focal gray matter atrophy responsible for oculomotor dysfunction. Significantly increased latency in the prosaccade task and significantly worse performance in the anti-saccade task were found in MS patients. The detailed examination of conjugated eye movements revealed five subclinical internuclear ophthalmoparesis cases. The peak velocity and latency of the anti-saccade movement correlated with the number of black holes, but none of the eye movement parameters were associated with the T2 lesion burden or location. Global gray matter volume correlated with saccade and anti-saccade latency, whereas white matter and total brain volume did not. Local gray matter atrophy in the left inferio-parietal lobule and temporo-occipital junction correlated with anti-saccade peak velocity. Our results show that neurodegeneration-like features of the MRI (black-hole, gray matter atrophy) are the best predictors of eye movement deficit in MS. Concurring with the clinico-radiological paradox, T2 lesion burden cannot explain the behavioral results. Importantly, anti-saccade peak velocity correlates with gray matter atrophy in the left parietal regions, which are frequently implicated in attention tasks.
Keywords: anti-saccade; atrophy; black hole; multiple sclerosis; prosaccade.
Figures

References
-
- Mastaglia FL, Black JL, Collins DW. Quantitative studies of saccadic and pursuit eye movements in multiple sclerosis. Brain. (1979) 102:817–34. - PubMed
LinkOut - more resources
Full Text Sources

