Thyroid Hormone Action on Innate Immunity
- PMID: 31214123
- PMCID: PMC6558108
- DOI: 10.3389/fendo.2019.00350
Thyroid Hormone Action on Innate Immunity
Erratum in
-
Corrigendum: Thyroid Hormone Action on Innate Immunity.Front Endocrinol (Lausanne). 2019 Jul 19;10:486. doi: 10.3389/fendo.2019.00486. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31379751 Free PMC article.
Abstract
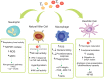
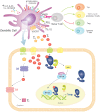
The interplay between thyroid hormone action and the immune system has been established in physiological and pathological settings. However, their connection is complex and still not completely understood. The thyroid hormones (THs), 3,3',5,5' tetraiodo-L-thyroxine (T4) and 3,3',5-triiodo-L-thyronine (T3) play essential roles in both the innate and adaptive immune responses. Despite much research having been carried out on this topic, the available data are sometimes difficult to interpret or even contradictory. Innate immune cells act as the first line of defense, mainly involving granulocytes and natural killer cells. In turn, antigen presenting cells, macrophages and dendritic cells capture, process and present antigens (self and foreign) to naïve T lymphocytes in secondary lymphoid tissues for the development of adaptive immunity. Here, we review the cellular and molecular mechanisms involved in T4 and T3 effects on innate immune cells. An overview of the state-of-the-art of TH transport across the target cell membrane, TH metabolism inside these cells, and the genomic and non-genomic mechanisms involved in the action of THs in the different innate immune cell subsets is included. The present knowledge of TH effects as well as the thyroid status on innate immunity helps to understand the complex adaptive responses achieved with profound implications in immunopathology, which include inflammation, cancer and autoimmunity, at the crossroads of the immune and endocrine systems.
Keywords: dendritic cells; innate immunity; macrophages; natural killer cells; neutrophils; thyroid hormones.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

