The Role of Antibodies and Their Receptors in Protection Against Ordered Protein Assembly in Neurodegeneration
- PMID: 31214163
- PMCID: PMC6554282
- DOI: 10.3389/fimmu.2019.01139
The Role of Antibodies and Their Receptors in Protection Against Ordered Protein Assembly in Neurodegeneration
Abstract
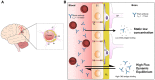
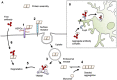
Ordered assemblies of proteins are found in the postmortem brains of sufferers of several neurodegenerative diseases. The cytoplasmic microtubule associated protein tau and alpha-synuclein (αS) are found in an assembled state in Alzheimer's disease and Parkinson's disease, respectively. An accumulating body of evidence suggests a "prion-like" mechanism of spread of these assemblies through the diseased brain. Under this hypothesis, assembled variants of these proteins promote the conversion of native proteins to the assembled state. This likely inflicts pathology on cells of the brain through a toxic gain-of-function mechanism. Experiments in animal models of tau and αS pathology have demonstrated that the passive transfer of anti-tau or anti-αS antibodies induces a reduction in the levels of assembled proteins. This is further accompanied by improvements in neurological function and preservation of brain volume. Immunotherapy is therefore considered one of the brightest hopes as a therapeutic avenue in an area currently without disease-modifying therapy. Following a series of disappointing clinical trials targeting beta-amyloid, a peptide that accumulates in the extracellular spaces of the AD brain, attention is turning to active and passive immunotherapies that target tau and αS. However, there are several remaining uncertainties concerning the mechanism by which antibodies afford protection against self-propagating protein conformations. This review will discuss current understanding of how antibodies and their receptors can be brought to bear on proteins involved in neurodegeneration. Parallels will be made to antibody-mediated protection against classical viral infections. Common mechanisms that may contribute to protection against self-propagating protein conformations include blocking the entry of protein "seeds" to cells, clearance of immune complexes by microglia, and the intracellular protein degradation pathway initiated by cytoplasmic antibodies via the Fc receptor TRIM21. As with anti-viral immunity, protective mechanisms may be accompanied by the activation of immune signaling pathways and we will discuss the suitability of such activation in the neurological setting.
Keywords: Fc receptor; alpha-synuclein; antibody immunity; beta-amyloid; microglia; neurodegeneration; prion-like proteins; tau (MAPT).
Figures

References
-
- About a peculiar disease of the cerebral cortex. By Alois Alzheimer, 1907 (Translated by Jarvik L., Greenson H.). Alzheimer Dis Assoc Disord. (1987) 1:3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

