High Salt Inhibits Tumor Growth by Enhancing Anti-tumor Immunity
- PMID: 31214164
- PMCID: PMC6557976
- DOI: 10.3389/fimmu.2019.01141
High Salt Inhibits Tumor Growth by Enhancing Anti-tumor Immunity
Abstract
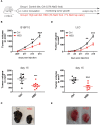
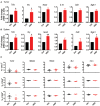
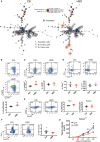
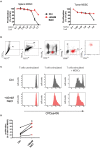
Excess salt intake could affect the immune system by shifting the immune cell balance toward a pro-inflammatory state. Since this shift of the immune balance is thought to be beneficial in anti-cancer immunity, we tested the impact of high salt diets on tumor growth in mice. Here we show that high salt significantly inhibited tumor growth in two independent murine tumor transplantation models. Although high salt fed tumor-bearing mice showed alterations in T cell populations, the effect seemed to be largely independent of adaptive immune cells. In contrast, depletion of myeloid-derived suppressor cells (MDSCs) significantly reverted the inhibitory effect on tumor growth. In line with this, high salt conditions almost completely blocked murine MDSC function in vitro. Importantly, similar effects were observed in human MDSCs isolated from cancer patients. Thus, high salt conditions seem to inhibit tumor growth by enabling more pronounced anti-tumor immunity through the functional modulation of MDSCs. Our findings might have critical relevance for cancer immunotherapy.
Keywords: MDSC; cancer; cancer immunotherapy; dietary factor; sodium chloride (dietary).
Figures

Similar articles
-
Myeloid Derived Suppressor Cells: Key Drivers of Immunosuppression in Ovarian Cancer.Front Immunol. 2019 Jun 4;10:1273. doi: 10.3389/fimmu.2019.01273. eCollection 2019. Front Immunol. 2019. PMID: 31214202 Free PMC article.
-
High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation.Nat Commun. 2020 Apr 7;11(1):1732. doi: 10.1038/s41467-020-15524-1. Nat Commun. 2020. PMID: 32265505 Free PMC article.
-
Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis.Cell Death Dis. 2017 May 11;8(5):e2779. doi: 10.1038/cddis.2017.192. Cell Death Dis. 2017. PMID: 28492541 Free PMC article.
-
Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment.J Immunol. 2018 Jan 15;200(2):422-431. doi: 10.4049/jimmunol.1701019. J Immunol. 2018. PMID: 29311384 Free PMC article. Review.
-
Relevance of Interferon Regulatory Factor-8 Expression in Myeloid-Tumor Interactions.J Interferon Cytokine Res. 2016 Jul;36(7):442-53. doi: 10.1089/jir.2015.0174. J Interferon Cytokine Res. 2016. PMID: 27379866 Free PMC article. Review.
Cited by
-
Cancer Acidity and Hypertonicity Contribute to Dysfunction of Tumor-Associated Dendritic Cells: Potential Impact on Antigen Cross-Presentation Machinery.Cancers (Basel). 2020 Aug 24;12(9):2403. doi: 10.3390/cancers12092403. Cancers (Basel). 2020. PMID: 32847079 Free PMC article. Review.
-
Low-Salt Diet Reduces Anti-CTLA4 Mediated Systemic Immune-Related Adverse Events while Retaining Therapeutic Efficacy against Breast Cancer.Biology (Basel). 2022 May 25;11(6):810. doi: 10.3390/biology11060810. Biology (Basel). 2022. PMID: 35741331 Free PMC article.
-
Chronic High-Salt Diet Activates Tumor-Initiating Stem Cells Leading to Breast Cancer Proliferation.Cells. 2024 May 25;13(11):912. doi: 10.3390/cells13110912. Cells. 2024. PMID: 38891044 Free PMC article.
-
High-Salt Tumor Microenvironment: Not as Bad as It Sounds, Not as Good as It Seems.Cancers (Basel). 2025 Jun 10;17(12):1924. doi: 10.3390/cancers17121924. Cancers (Basel). 2025. PMID: 40563574 Free PMC article. Review.
-
Impact of high-salt diet in health and diseases and its role in pursuit of cancer immunotherapy by modulating gut microbiome.J Family Med Prim Care. 2024 May;13(5):1628-1635. doi: 10.4103/jfmpc.jfmpc_1574_23. Epub 2024 May 24. J Family Med Prim Care. 2024. PMID: 38948582 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

