Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells
- PMID: 31214189
- PMCID: PMC6558076
- DOI: 10.3389/fimmu.2019.01235
Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells
Abstract
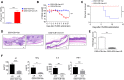
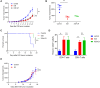
Immune checkpoint blockade (ICB) immunotherapy increases antitumor immunity by blocking cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4) or programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) and displays robust clinical responses in various cancers. However, ICB immunotherapy also triggers severe inflammatory side effects, known as immune-related adverse effects (irAEs). One of the most common toxicities is immune checkpoint blockade-associated colitis (ICB associated colitis). The exact mechanism of ICB associated colitis remains to be explored. Here, we combined ICB (anti-CTLA-4 and anti-PD-1) treatment with a standard colitis model, in which a more severe form of colitis is induced in mice, to recapitulate the clinical observations in patients receiving combined ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) therapy, during which colitis is the most frequent complication encountered. We found that the composition of the gut microbiota changed in ICB associated colitis. Principal component analysis of the gut microbiome showed an obvious reduction in the abundance of Lactobacillus in severe ICB associated colitis. Lactobacillus depletion completely by vancomycin augmented the immunopathology of ICB. Furthermore, we found that the ICB toxicity could be totally eliminated via the administration of a widely available probiotic Lactobacillus reuteri (L.reuteri). Oral administration of L. reuteri therapeutically inhibited the development and progression of colitis, thus ameliorating the loss of body weight and inflammatory status induced by ICB treatment. Mechanistically, the protective effect of L. reuteri was associated with a decrease in the distribution of group 3 innate lymphocytes (ILC3s) induced by ICB associated colitis. In conclusion, our study highlights the immunomodulatory mechanism of the gut microbiota and suggests that manipulating the gut microbiota by administrating L. reuteri can mitigate the autoimmunity induced by ICB, thus allowing ICB immunotherapy to stimulate the desired immune response without an apparent immunopathology.
Keywords: colitis; gut microbiome; immune checkpoint blockade; inflammation; innate lymphoid cells.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

