Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-γ Production
- PMID: 31214198
- PMCID: PMC6558015
- DOI: 10.3389/fimmu.2019.01261
Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-γ Production
Abstract
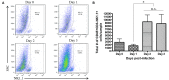
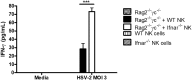
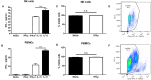
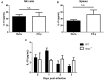
NK cells are a key antiviral component of the innate immune response to HSV-2, particularly through their production of IFN-γ. It is still commonly thought that type I IFN activates NK cell function; however, rather than requiring the type I IFN receptor themselves, we have previously found that type I IFN activates NK cells through an indirect mechanism involving inflammatory monocytes and IL-18. Here, we further show that direct action of type I IFN on NK cells, rather than inducing IFN-γ, negatively regulates its production during HSV-2 infection and cytokine stimulation. During infection, IFN-γ is rapidly induced from NK cells at day 2 post-infection and then immediately downregulated at day 3 post-infection. We found that this downregulation of IFN-γ release was not due to a loss of NK cells at day 3 post-infection, but negatively regulated through IFN signaling on NK cells. Absence of IFNAR on NK cells led to a significantly increased level of IFN-γ compared to WT NK cells after HSV-2 infection in vitro. Further, priming of NK cells with type I IFN was able to suppress cytokine-induced IFN-γ production from both human and mouse NK cells. We found that this immunosuppression was not mediated by IL-10. Rather, we found that type I IFN induced a significant increase in Axl expression on human NK cells. Overall, our data suggests that type I IFN negatively regulates NK cell IFN-γ production through a direct mechanism in vitro and during HSV-2 infection.
Keywords: HSV; Human NK cells; IFN-γ; NK cells; type I IFN.
Figures



Similar articles
-
Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection.J Exp Med. 2017 Apr 3;214(4):1153-1167. doi: 10.1084/jem.20160880. Epub 2017 Mar 6. J Exp Med. 2017. PMID: 28264883 Free PMC article.
-
Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response.J Immunol. 1999 Apr 15;162(8):4511-20. J Immunol. 1999. PMID: 10201989
-
NK cells require type I IFN receptor for antiviral responses during genital HSV-2 infection.Cell Immunol. 2011;269(1):29-37. doi: 10.1016/j.cellimm.2011.03.007. Epub 2011 Mar 12. Cell Immunol. 2011. PMID: 21477795
-
Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts).Clin Ter. 2006 Jul-Aug;157(4):377-86. Clin Ter. 2006. Retraction in: Clin Ter. 2008 May-Jun;159(3):207. PMID: 17051976 Retracted. Review.
-
Metabolic regulation of NK cell function: implications for immunotherapy.Immunometabolism (Cobham). 2023 Jan 23;5(1):e00020. doi: 10.1097/IN9.0000000000000020. eCollection 2023 Jan. Immunometabolism (Cobham). 2023. PMID: 36710923 Free PMC article. Review.
Cited by
-
Single cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets.Nat Commun. 2023 Nov 15;14(1):7387. doi: 10.1038/s41467-023-43181-7. Nat Commun. 2023. PMID: 37968278 Free PMC article.
-
IFNAR2 Deficiency Causing Dysregulation of NK Cell Functions and Presenting With Hemophagocytic Lymphohistiocytosis.Front Genet. 2020 Sep 18;11:937. doi: 10.3389/fgene.2020.00937. eCollection 2020. Front Genet. 2020. PMID: 33193576 Free PMC article.
-
Attenuating the Effects of Novel COVID-19 (SARS-CoV-2) Infection-Induced Cytokine Storm and the Implications.J Inflamm Res. 2021 Apr 16;14:1487-1510. doi: 10.2147/JIR.S301784. eCollection 2021. J Inflamm Res. 2021. PMID: 33889008 Free PMC article. Review.
-
TAM Receptor Inhibition-Implications for Cancer and the Immune System.Cancers (Basel). 2021 Mar 10;13(6):1195. doi: 10.3390/cancers13061195. Cancers (Basel). 2021. PMID: 33801886 Free PMC article. Review.
-
Therapeutic Options for Coronavirus Disease 2019 (COVID-19) - Modulation of Type I Interferon Response as a Promising Strategy?Front Public Health. 2020 May 15;8:185. doi: 10.3389/fpubh.2020.00185. eCollection 2020. Front Public Health. 2020. PMID: 32574289 Free PMC article. No abstract available.
References
-
- Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, et al. . NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. (2012) 36:1047–59. 10.1016/j.immuni.2012.03.026 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

