Genetic Susceptibility to Chronic Kidney Disease - Some More Pieces for the Heritability Puzzle
- PMID: 31214239
- PMCID: PMC6554557
- DOI: 10.3389/fgene.2019.00453
Genetic Susceptibility to Chronic Kidney Disease - Some More Pieces for the Heritability Puzzle
Abstract
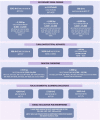
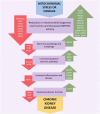
Chronic kidney disease (CKD) is a major global health problem with an increasing prevalence partly driven by aging population structure. Both genomic and environmental factors contribute to this complex heterogeneous disease. CKD heritability is estimated to be high (30-75%). Genome-wide association studies (GWAS) and GWAS meta-analyses have identified several genetic loci associated with CKD, including variants in UMOD, SHROOM3, solute carriers, and E3 ubiquitin ligases. However, these genetic markers do not account for all the susceptibility to CKD, and the causal pathways remain incompletely understood; other factors must be contributing to the missing heritability. Less investigated biological factors such as telomere length; mitochondrial proteins, encoded by nuclear genes or specific mitochondrial DNA (mtDNA) encoded genes; structural variants, such as copy number variants (CNVs), insertions, deletions, inversions and translocations are poorly covered and may explain some of the missing heritability. The sex chromosomes, often excluded from GWAS studies, may also help explain gender imbalances in CKD. In this review, we outline recent findings on molecular biomarkers for CKD (telomeres, CNVs, mtDNA variants, sex chromosomes) that typically have received less attention than gene polymorphisms. Shorter telomere length has been associated with renal dysfunction and CKD progression, however, most publications report small numbers of subjects with conflicting findings. CNVs have been linked to congenital anomalies of the kidney and urinary tract, posterior urethral valves, nephronophthisis and immunoglobulin A nephropathy. Information on mtDNA biomarkers for CKD comes primarily from case reports, therefore the data are scarce and diverse. The most consistent finding is the A3243G mutation in the MT-TL1 gene, mainly associated with focal segmental glomerulosclerosis. Only one GWAS has found associations between X-chromosome and renal function (rs12845465 and rs5987107). No loci in the Y-chromosome have reached genome-wide significance. In conclusion, despite the efforts to find the genetic basis of CKD, it remains challenging to explain all of the heritability with currently available methods and datasets. Although additional biomarkers have been investigated in less common suspects such as telomeres, CNVs, mtDNA and sex chromosomes, hidden heritability in CKD remains elusive, and more comprehensive approaches, particularly through the integration of multiple -"omics" data, are needed.
Keywords: chronic kidney disease; copy number variants; mitochondria; single nucleotide polymorphisms; telomeres; whole exome sequencing.
Figures

Similar articles
-
Genetic variants affecting mitochondrial function provide further insights for kidney disease.BMC Genomics. 2024 Jun 10;25(1):576. doi: 10.1186/s12864-024-10449-1. BMC Genomics. 2024. PMID: 38858654 Free PMC article.
-
Genome-Wide Association Study of Renal Function Traits: Results from the Japan Multi-Institutional Collaborative Cohort Study.Am J Nephrol. 2018;47(5):304-316. doi: 10.1159/000488946. Epub 2018 May 18. Am J Nephrol. 2018. PMID: 29779033
-
Mitochondrial DNA Variants at Low-Level Heteroplasmy and Decreased Copy Numbers in Chronic Kidney Disease (CKD) Tissues with Kidney Cancer.Int J Mol Sci. 2023 Dec 7;24(24):17212. doi: 10.3390/ijms242417212. Int J Mol Sci. 2023. PMID: 38139039 Free PMC article.
-
Chronic kidney disease: novel insights from genome-wide association studies.Kidney Blood Press Res. 2011;34(4):225-34. doi: 10.1159/000326901. Epub 2011 Jun 21. Kidney Blood Press Res. 2011. PMID: 21691125 Review.
-
Genome-wide studies to identify risk factors for kidney disease with a focus on patients with diabetes.Nephrol Dial Transplant. 2015 Aug;30 Suppl 4:iv26-34. doi: 10.1093/ndt/gfv087. Nephrol Dial Transplant. 2015. PMID: 26209735 Review.
Cited by
-
Diagnostic Yield of Next-Generation Sequencing in Patients With Chronic Kidney Disease of Unknown Etiology.Front Genet. 2019 Dec 13;10:1264. doi: 10.3389/fgene.2019.01264. eCollection 2019. Front Genet. 2019. PMID: 31921302 Free PMC article. Review.
-
Meta-analysis of African ancestry genome-wide association studies identified novel locus and validates multiple loci associated with kidney function.BMC Genomics. 2023 Aug 29;24(1):496. doi: 10.1186/s12864-023-09601-0. BMC Genomics. 2023. PMID: 37644460 Free PMC article.
-
Mechanisms of ferroptosis in chronic kidney disease.Front Mol Biosci. 2022 Aug 24;9:975582. doi: 10.3389/fmolb.2022.975582. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090053 Free PMC article. Review.
-
Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors.Int J Mol Sci. 2021 May 28;22(11):5808. doi: 10.3390/ijms22115808. Int J Mol Sci. 2021. PMID: 34071671 Free PMC article. Review.
-
Limited clinical utility for GWAS or polygenic risk score for postoperative acute kidney injury in non-cardiac surgery in European-ancestry patients.BMC Nephrol. 2022 Oct 21;23(1):339. doi: 10.1186/s12882-022-02964-8. BMC Nephrol. 2022. PMID: 36271344 Free PMC article.
References
-
- Adema A. Y., Janssen M. C. H., van der Heijden J. W. (2016). A novel mutation in mitochondrial DNA in a patient with diabetes, deafness and proteinuria. Neth. J. Med. 74 455–457. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

