5-Azacytidine and Resveratrol Enhance Chondrogenic Differentiation of Metabolic Syndrome-Derived Mesenchymal Stem Cells by Modulating Autophagy
- PMID: 31214275
- PMCID: PMC6535830
- DOI: 10.1155/2019/1523140
5-Azacytidine and Resveratrol Enhance Chondrogenic Differentiation of Metabolic Syndrome-Derived Mesenchymal Stem Cells by Modulating Autophagy
Abstract
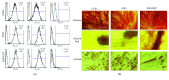
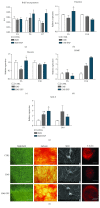
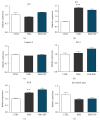
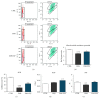
Recently, metabolic syndrome (MS) has gained attention in human and animal metabolic medicine. Insulin resistance, inflammation, hyperleptinemia, and hyperinsulinemia are critical to its definition. MS is a complex cluster of metabolic risk factors that together exert a wide range of effects on multiple organs, tissues, and cells in the body. Adipose stem cells (ASCs) are multipotent stem cell population residing within the adipose tissue that is inflamed during MS. Studies have indicated that these cells lose their stemness and multipotency during MS, which strongly reduces their therapeutic potential. They suffer from oxidative stress, apoptosis, and mitochondrial deterioration. Thus, the aim of this study was to rejuvenate these cells in vitro in order to improve their chondrogenic differentiation effectiveness. Pharmacotherapy of ASCs was based on resveratrol and 5-azacytidine pretreatment. We evaluated whether those substances are able to reverse aged phenotype of metabolic syndrome-derived ASCs and improve their chondrogenic differentiation at its early stage using immunofluorescence, transmission and scanning electron microscopy, real-time PCR, and flow cytometry. Obtained results indicated that 5-azacytidine and resveratrol modulated mitochondrial dynamics, autophagy, and ER stress, leading to the enhancement of chondrogenesis in metabolically impaired ASCs. Therefore, pretreatment of these cells with 5-azacytidine and resveratrol may become a necessary intervention before clinical application of these cells in order to strengthen their multipotency and therapeutic potential.
Figures



Similar articles
-
Combination of resveratrol and 5-azacytydine improves osteogenesis of metabolic syndrome mesenchymal stem cells.J Cell Mol Med. 2018 Oct;22(10):4771-4793. doi: 10.1111/jcmm.13731. Epub 2018 Jul 12. J Cell Mol Med. 2018. PMID: 29999247 Free PMC article.
-
5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy.J Cell Mol Med. 2019 Jan;23(1):237-259. doi: 10.1111/jcmm.13914. Epub 2018 Oct 28. J Cell Mol Med. 2019. PMID: 30370650 Free PMC article.
-
Extracellular Microvesicles (MV's) Isolated from 5-Azacytidine-and-Resveratrol-Treated Cells Improve Viability and Ameliorate Endoplasmic Reticulum Stress in Metabolic Syndrome Derived Mesenchymal Stem Cells.Stem Cell Rev Rep. 2020 Dec;16(6):1343-1355. doi: 10.1007/s12015-020-10035-4. Stem Cell Rev Rep. 2020. PMID: 32880856 Free PMC article.
-
Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use.Stem Cell Rev Rep. 2018 Jun;14(3):337-345. doi: 10.1007/s12015-018-9809-x. Stem Cell Rev Rep. 2018. PMID: 29611042 Free PMC article. Review.
-
Insight into the development of obesity: functional alterations of adipose-derived mesenchymal stem cells.Obes Rev. 2018 Jul;19(7):888-904. doi: 10.1111/obr.12679. Epub 2018 Mar 8. Obes Rev. 2018. PMID: 29521029 Review.
Cited by
-
miR-26a-5p Suppresses Wnt/β-Catenin Signaling Pathway by Inhibiting DNMT3A-Mediated SFRP1 Methylation and Inhibits Cancer Stem Cell-Like Properties of NSCLC.Dis Markers. 2022 Jul 11;2022:7926483. doi: 10.1155/2022/7926483. eCollection 2022. Dis Markers. 2022. PMID: 35860691 Free PMC article.
-
Mitochondrial Dynamics: Fission and Fusion in Fate Determination of Mesenchymal Stem Cells.Front Cell Dev Biol. 2020 Oct 15;8:580070. doi: 10.3389/fcell.2020.580070. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33178694 Free PMC article. Review.
-
MSI-1436 improves EMS adipose derived progenitor stem cells in the course of adipogenic differentiation through modulation of ER stress, apoptosis, and oxidative stress.Stem Cell Res Ther. 2021 Feb 3;12(1):97. doi: 10.1186/s13287-020-02102-x. Stem Cell Res Ther. 2021. PMID: 33536069 Free PMC article.
-
Evaluation of Autophagy Process in Differentiation of Human Induced Pluripotent Stem Cells toward Insulin Producing Cells.Int J Organ Transplant Med. 2022;13(2):4-13. Int J Organ Transplant Med. 2022. PMID: 37641731 Free PMC article.
-
Effects of resveratrol and its analogues on the cell cycle of equine mesenchymal stem/stromal cells.J Equine Sci. 2023 Sep;34(3):67-72. doi: 10.1294/jes.34.67. Epub 2023 Sep 8. J Equine Sci. 2023. PMID: 37781569 Free PMC article.
References
-
- Frank N. Equine metabolic syndrome. Journal of Equine Veterinary Science. 2009;29(5):259–267. doi: 10.1016/j.jevs.2009.04.183. - DOI
-
- Marycz K., Kornicka K., Szlapka-Kosarzewska J., Weiss C. Excessive endoplasmic reticulum stress correlates with impaired mitochondrial dynamics, mitophagy and apoptosis, in liver and adipose tissue, but not in muscles in EMS horses. International Journal of Molecular Sciences. 2018;19(1):p. 165. doi: 10.3390/ijms19010165. - DOI - PMC - PubMed
-
- Mendes A. F., Rosa S. C., Rufino A. T., Ribeiro M., Judas F. Diabetes-induced osteoarthritis: role of hyperglycemia in joint destruction. BMC Musculoskeletal Disorders. 2015;16(S1) Supplement 1 doi: 10.1186/1471-2474-16-S1-S1. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

