Recent trends in prenatal genetic screening and testing
- PMID: 31214330
- PMCID: PMC6545823
- DOI: 10.12688/f1000research.16837.1
Recent trends in prenatal genetic screening and testing
Abstract
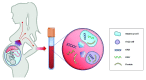
Prenatal testing in recent years has been moving toward non-invasive methods to determine the fetal risk for genetic disorders without incurring the risk of miscarriage. Rapid progress of modern high-throughput molecular technologies along with the discovery of cell-free fetal DNA in maternal plasma led to novel screening methods for fetal chromosomal aneuploidies. Such tests are referred to as non-invasive prenatal tests (NIPTs), non-invasive prenatal screening, or prenatal cell-free DNA screening. Owing to many advantages, the adoption of NIPT in routine clinical practice was very rapid and global. As an example, NIPT has recently become a standard screening procedure for all pregnant women in the Netherlands. On the other hand, invasive sampling procedures remain important, especially for their diagnostic value in the confirmation of NIPT-positive findings and the detection of Mendelian disorders. In this review, we focus on current trends in the field of NIPT and discuss their benefits, drawbacks, and consequences in regard to routine diagnostics.
Keywords: NIPT; amniocentesis; cffDNA; fetal aneuploidies; non-invasive; screening.
Conflict of interest statement
Competing interests: TS and JB work for the company Geneton, which has developed a whole genome base NIPT test which is used in clinical practice in Slovakia. OP has no competing interests.No competing interests were disclosed.No competing interests were disclosed.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

