The gelling effect of platelet-rich fibrin matrix when exposed to human tenocytes from the rotator cuff in small-diameter culture wells and the design of a co-culture device to overcome this phenomenon
- PMID: 31214334
- PMCID: PMC6549006
- DOI: 10.1302/2046-3758.85.BJR-2018-0258.R1
The gelling effect of platelet-rich fibrin matrix when exposed to human tenocytes from the rotator cuff in small-diameter culture wells and the design of a co-culture device to overcome this phenomenon
Abstract
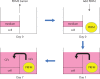
Objectives: Platelet-rich fibrin matrix (PRFM) has been proved to enhance tenocyte proliferation but has mixed results when used during rotator cuff repair. The optimal PRFM preparation protocol should be determined before clinical application. To screen the best PRFM to each individual's tenocytes effectively, small-diameter culture wells should be used to increase variables. The gelling effect of PRFM will occur when small-diameter culture wells are used. A co-culture device should be designed to avoid this effect.
Methods: Tenocytes harvested during rotator cuff repair and blood from a healthy volunteer were used. Tenocytes were seeded in 96-, 24-, 12-, and six-well plates and co-culture devices. Appropriate volumes of PRFM, according to the surface area of each culture well, were treated with tenocytes for seven days. The co-culture device was designed to avoid the gelling effect that occurred in the small-diameter culture well. Cell proliferation was analyzed by water soluble tetrazolium-1 (WST-1) bioassay.
Results: The relative quantification (condition/control) of WST-1 assay on day seven revealed a significant decrease in tenocyte proliferation in small-diameter culture wells (96 and 24 wells) due to the gelling effect. PRFM in large-diameter culture wells (12 and six wells) and co-culture systems induced a significant increase in tenocyte proliferation compared with the control group. The gelling effect of PRFM was avoided by the co-culture device.
Conclusion: When PRFM and tenocytes are cultured in small-diameter culture wells, the gelling effect will occur and make screening of personalized best-fit PRFM difficult. This effect can be avoided with the co-culture device.Cite this article: C-H. Chiu, P. Chen, W-L. Yeh, A. C-Y. Chen, Y-S. Chan, K-Y. Hsu, K-F. Lei. The gelling effect of platelet-rich fibrin matrix when exposed to human tenocytes from the rotator cuff in small-diameter culture wells and the design of a co-culture device to overcome this phenomenon. Bone Joint Res 2019;8:216-223. DOI: 10.1302/2046-3758.85.BJR-2018-0258.R1.
Keywords: Co-culture; Gelling effect; Platelet-rich fibrin matrix; Rotator cuff tears; Tenocytes.
Conflict of interest statement
Conflict of interest statement: All the authors report institutional grants (paid to Chang Gung Memorial Hospital, Linkou, Taiwan) from Chang Gung Memorial Hospital, Linkou, Taiwan and the Taiwan Minister of Science and Technology. There were no competing interests among all authors.
Figures




Similar articles
-
The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study.Am J Sports Med. 2012 Jun;40(6):1234-41. doi: 10.1177/0363546512442924. Epub 2012 Apr 10. Am J Sports Med. 2012. PMID: 22495146 Clinical Trial.
-
Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears.Am J Sports Med. 2012 Feb;40(2):286-93. doi: 10.1177/0363546511424402. Epub 2011 Oct 20. Am J Sports Med. 2012. PMID: 22016459
-
Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study.Am J Sports Med. 2013 Feb;41(2):263-70. doi: 10.1177/0363546512467621. Epub 2012 Nov 30. Am J Sports Med. 2013. PMID: 23204506 Clinical Trial.
-
Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions.Curr Pharm Biotechnol. 2012 Jun;13(7):1196-206. doi: 10.2174/138920112800624337. Curr Pharm Biotechnol. 2012. PMID: 21740372 Review.
-
Arthroscopic Rotator Cuff Repair: A Systematic Review of Overlapping Meta-Analyses.JBJS Rev. 2019 Apr;7(4):e1. doi: 10.2106/JBJS.RVW.18.00027. JBJS Rev. 2019. PMID: 30939497
Cited by
-
Leukocyte-Rich Platelet-Rich Plasma Has Better Stimulating Effects on Tenocyte Proliferation Compared With Leukocyte-Poor Platelet-Rich Plasma.Orthop J Sports Med. 2022 Mar 15;10(3):23259671221084706. doi: 10.1177/23259671221084706. eCollection 2022 Mar. Orthop J Sports Med. 2022. PMID: 35309233 Free PMC article.
-
Personalized, Predictive, Participatory, Precision, and Preventive (P5) Medicine in Rotator Cuff Tears.J Pers Med. 2021 Apr 1;11(4):255. doi: 10.3390/jpm11040255. J Pers Med. 2021. PMID: 33915689 Free PMC article.
-
Advances in the application of hydrogel-based scaffolds for tendon repair.Genes Dis. 2023 Jul 7;11(4):101019. doi: 10.1016/j.gendis.2023.04.039. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38560496 Free PMC article. Review.
-
Application of Platelet-Rich Plasma in Arthroscopic Rotator Cuff Repair: A Systematic Review and Meta-analysis.Orthop J Sports Med. 2021 Jul 13;9(7):23259671211016847. doi: 10.1177/23259671211016847. eCollection 2021 Jul. Orthop J Sports Med. 2021. PMID: 34345632 Free PMC article. Review.
-
The inadequate reporting of sex in research.Bone Joint Res. 2020 Oct;9(10):729-730. doi: 10.1302/2046-3758.910.BJR-2020-0351.R1. Bone Joint Res. 2020. PMID: 33399472 Free PMC article. No abstract available.
References
-
- Wong CC, Chen CH, Chan WP, et al. Single-stage cartilage repair using platelet-rich fibrin scaffolds with autologous cartilaginous grafts. Am J Sports Med 2017;45:3128-3142. - PubMed
-
- Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med 2011;39:258-265. - PubMed
-
- Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg [Am] 2012;94-A:1345-1352. - PubMed
-
- Rodeo SA, Delos D, Williams RJ, et al. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med 2012;40:1234-1241. - PubMed

