Synthesis and Structures of Ruthenium Carbonyl Complexes Bearing Pyridine-Alkoxide Ligands and Their Catalytic Activity in Alcohol Oxidation
- PMID: 31214574
- PMCID: PMC6558070
- DOI: 10.3389/fchem.2019.00394
Synthesis and Structures of Ruthenium Carbonyl Complexes Bearing Pyridine-Alkoxide Ligands and Their Catalytic Activity in Alcohol Oxidation
Abstract
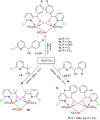
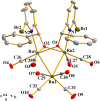
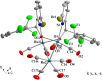
Reaction of Ru3(CO)12 with two equiv of 6-bromopyridine alcohols 6-bromopyCHROH [(R = C6H5 (L1); R = 4-CH3C6H4 (L2); R = 4-OMeC6H4 (L3); R = 4-ClC6H4 (L4); (R = 4-CF3C6H4 (L5); R = 2-OMeC6H4 (L6); R = 2-CF3C6H4 (L7)] and 6-bromopyC(Me)2OH (L8) in refluxing xylene afforded novel trinuclear ruthenium complexes [6-bromopyCHRO]2Ru3(CO)8 (1a-1g) and [6-bromopyC(Me)2O]2Ru3(CO)8 (1h). These complexes were characterized by FT-IR and NMR spectroscopy as well as elemental analysis. The structures of all the complexes were further confirmed by X-ray crystallographic analysis. In the presence of tert-butyl hydroperoxide (TBHP) as the source of oxidant, complexes 1a-1h displayed high catalytic activities for oxidation of primary and secondary alcohols and most of oxidation reactions could be completed within 1 h at room temperature.
Keywords: alcohols oxidation; chemoselectivity; pyridine alcohols; ruthenium carbonyl complexes; t-butyl hydroperoxide.
Figures



Similar articles
-
Aerobic oxidation of primary benzylic amines to amides and nitriles catalyzed by ruthenium carbonyl clusters carrying N,O-bidentate ligands.Dalton Trans. 2020 Mar 21;49(11):3480-3487. doi: 10.1039/d0dt00045k. Epub 2020 Feb 27. Dalton Trans. 2020. PMID: 32103217
-
Preparation of the Ru3(CO)8-pyridine-alcohol cluster and its use for the selective catalytic transformation of primary to secondary amines.Dalton Trans. 2018 Oct 9;47(39):14033-14040. doi: 10.1039/c8dt02972e. Dalton Trans. 2018. PMID: 30232491
-
Fluorous biphasic catalysis: synthesis and characterization of copper(I) and copper(II) fluoroponytailed 1,4,7-Rf-TACN and 2,2'-Rf-bipyridine complexes--their catalytic activity in the oxidation of hydrocarbons, olefins, and alcohols, including mechanistic implications.Chemistry. 2003 Sep 5;9(17):4168-78. doi: 10.1002/chem.200304771. Chemistry. 2003. PMID: 12953202
-
Syntheses of 8-quinolinolatocobalt(III) complexes containing cyclen based auxiliary ligands as models for hypoxia-activated prodrugs.Dalton Trans. 2010 Dec 28;39(48):11535-50. doi: 10.1039/c0dt01142h. Epub 2010 Nov 22. Dalton Trans. 2010. PMID: 21103540
-
Novel pyrazolylphosphite- and pyrazolylphosphinite-ruthenium(ii) complexes as catalysts for hydrogenation of acetophenone.Dalton Trans. 2016 Sep 14;45(34):13514-24. doi: 10.1039/c6dt02164f. Epub 2016 Aug 9. Dalton Trans. 2016. PMID: 27504937
References
-
- Annunziata A., Esposito R., Gatto G., Cucciolito M. E., Tuzi A., Macchioni A., et al. (2018). Iron (III) complexes with cross-bridged cyclams: synthesis and use in alcohol and water oxidation catalysis. Eur. J. Inorg. Chem. 2018, 3304–3311. 10.1002/ejic.201800451 - DOI
-
- Borah B. J., Mahanta A., Mondal M., Gogoi H., Yamada Y., Bharali P. (2018). Cobalt-copper nanoparticles catalyzed selective oxidation reactions: efficient catalysis at room temperature. Chem. Select 3, 9826–9832. 10.1002/slct.201801140 - DOI
LinkOut - more resources
Full Text Sources

