At the Intersection of Biomaterials and Gene Therapy: Progress in Non-viral Delivery of Nucleic Acids
- PMID: 31214586
- PMCID: PMC6558074
- DOI: 10.3389/fbioe.2019.00131
At the Intersection of Biomaterials and Gene Therapy: Progress in Non-viral Delivery of Nucleic Acids
Abstract
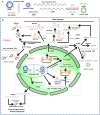
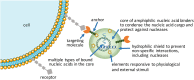
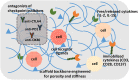
Biomaterials play a critical role in technologies intended to deliver therapeutic agents in clinical settings. Recent explosion of our understanding of how cells utilize nucleic acids has garnered excitement to develop a range of older (e.g., antisense oligonucleotides, plasmid DNA and transposons) and emerging (e.g., short interfering RNA, messenger RNA and non-coding RNAs) nucleic acid agents for therapy of a wide range of diseases. This review will summarize biomaterials-centered advances to undertake effective utilization of nucleic acids for therapeutic purposes. We first review various types of nucleic acids and their unique abilities to deliver a range of clinical outcomes. Using recent advances in T-cell based therapy as a case in point, we summarize various possibilities for utilizing biomaterials to make an impact in this exciting therapeutic intervention technology, with the belief that this modality will serve as a therapeutic paradigm for other types of cellular therapies in the near future. We subsequently focus on contributions of biomaterials in emerging nucleic acid technologies, specifically focusing on the design of intelligent nanoparticles, deployment of mRNA as an alternative to plasmid DNA, long-acting (integrating) expression systems, and in vitro/in vivo expansion of engineered T-cells. We articulate the role of biomaterials in these emerging nucleic acid technologies in order to enhance the clinical impact of nucleic acids in the near future.
Keywords: T-cell therapy; biomaterials; gene medicine; mRNA; nanoparticle; nucleic acid delivery; pDNA delivery; siRNA.
Figures
Similar articles
-
Advances in Nucleic Acid Research: Exploring the Potential of Oligonucleotides for Therapeutic Applications and Biological Studies.Int J Mol Sci. 2023 Dec 21;25(1):146. doi: 10.3390/ijms25010146. Int J Mol Sci. 2023. PMID: 38203317 Free PMC article. Review.
-
Nucleic acid-based therapeutics for dermal wound healing.Int J Biol Macromol. 2022 Nov 1;220:920-933. doi: 10.1016/j.ijbiomac.2022.08.099. Epub 2022 Aug 18. Int J Biol Macromol. 2022. PMID: 35987365 Review.
-
Biomaterial-based delivery systems of nucleic acid for regenerative research and regenerative therapy.Regen Ther. 2019 Jul 11;11:123-130. doi: 10.1016/j.reth.2019.06.007. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31338391 Free PMC article. Review.
-
Delivery of nucleic acid therapeutics for cancer immunotherapy.Med Drug Discov. 2020 Jun;6:100023. doi: 10.1016/j.medidd.2020.100023. Epub 2020 Mar 24. Med Drug Discov. 2020. PMID: 34337382 Free PMC article.
-
Non-viral nucleic acid containing nanoparticles as cancer therapeutics.Expert Opin Drug Deliv. 2016 Oct;13(10):1475-87. doi: 10.1080/17425247.2016.1190707. Epub 2016 Jun 6. Expert Opin Drug Deliv. 2016. PMID: 27248202 Free PMC article. Review.
Cited by
-
Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches-An Overview.Pharmaceutics. 2020 Aug 10;12(8):752. doi: 10.3390/pharmaceutics12080752. Pharmaceutics. 2020. PMID: 32785171 Free PMC article. Review.
-
mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles.Int J Mol Sci. 2024 Feb 1;25(3):1739. doi: 10.3390/ijms25031739. Int J Mol Sci. 2024. PMID: 38339015 Free PMC article. Review.
-
Sample preparation strategies for efficient correlation of 3D SIM and soft X-ray tomography data at cryogenic temperatures.Nat Protoc. 2021 Jun;16(6):2851-2885. doi: 10.1038/s41596-021-00522-4. Epub 2021 May 14. Nat Protoc. 2021. PMID: 33990802
-
Peptide-Assisted Nucleic Acid Delivery Systems on the Rise.Int J Mol Sci. 2021 Aug 23;22(16):9092. doi: 10.3390/ijms22169092. Int J Mol Sci. 2021. PMID: 34445799 Free PMC article. Review.
-
Insights on the DNA Stability in Aqueous Solutions of Ionic Liquids.Front Bioeng Biotechnol. 2020 Oct 14;8:547857. doi: 10.3389/fbioe.2020.547857. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33178668 Free PMC article.
References
-
- Aartsma-Rus A., Straub V., Hemmings R., Haas M., Schlosser-Weber G., Stoyanova-Beninska V., et al. . (2017). Development of exon skipping therapies for Duchenne Muscular Dystrophy: a critical review and a perspective on the outstanding issues. Nucleic Acid Ther. 27, 251–259. 10.1089/nat.2017.0682 - DOI - PMC - PubMed
-
- Abera G., Berhanu G., Tekewe A. (2012). Ribozymes: nucleic acid enzymes with potential pharmaceutical applications: a review. Pharmacophore 3, 164–178.
-
- Amante D. H., Smith T. R., Mendoza J. M., Schultheis K., McCoy J. R., Khan A. S., et al. . (2015). Skin transfection patterns and expression kinetics of electroporation-enhanced plasmid delivery using the CELLECTRA-3P, a portable next-generation dermal electroporation device. Hum. Gene Ther. Methods 26, 134–146. 10.1089/hgtb.2015.020 - DOI - PMC - PubMed
-
- American Association for Cancer Research (2017). Engineering CAR T cells with biomaterials. Cancer Discov. 7, 656–657. 10.1158/2159-8290.CD-NB2017-068 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

