Sjogren's Syndrome and TAM Receptors: A Possible Contribution to Disease Onset
- PMID: 31214622
- PMCID: PMC6535826
- DOI: 10.1155/2019/4813795
Sjogren's Syndrome and TAM Receptors: A Possible Contribution to Disease Onset
Abstract
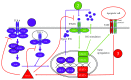
Sjogren's syndrome (SS) is a chronic, progressive autoimmune disease featuring both organ-specific and systemic manifestations, the most frequent being dry mouth and dry eyes resulting from lymphocytic infiltration into the salivary and lacrimal glands. Like the related autoimmune disease systemic lupus erythematosus (SLE), SS patients and mouse models display accumulation of apoptotic cells and a Type I interferon (IFN) signature. Receptor tyrosine kinases (RTKs) of the Tyro3, Axl, and Mer (TAM) family are present on the surface of macrophages and dendritic cells and participate in phagocytosis of apoptotic cells (efferocytosis) and inhibition of Type I IFN signaling. This review examines the relationship between TAM receptor dysfunction and SS and explores the potential contributions of TAM defects on macrophages to SS development.
Figures

Similar articles
-
Defective Efferocytosis in a Murine Model of Sjögren's Syndrome Is Mediated by Dysfunctional Mer Tyrosine Kinase Receptor.Int J Mol Sci. 2021 Sep 8;22(18):9711. doi: 10.3390/ijms22189711. Int J Mol Sci. 2021. PMID: 34575873 Free PMC article.
-
Clinical significance of TAM receptor in the minor salivary glands of patients with Sjögren's disease.Sci Rep. 2025 Jul 2;15(1):23065. doi: 10.1038/s41598-025-08086-z. Sci Rep. 2025. PMID: 40595270 Free PMC article.
-
The association of Tyro3/Axl/Mer signaling with inflammatory response, disease activity in patients with primary Sjögren's syndrome.Joint Bone Spine. 2015 Jul;82(4):258-63. doi: 10.1016/j.jbspin.2015.01.008. Epub 2015 Apr 13. Joint Bone Spine. 2015. PMID: 25881761
-
Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment.Mol Cancer. 2019 May 14;18(1):94. doi: 10.1186/s12943-019-1022-2. Mol Cancer. 2019. PMID: 31088471 Free PMC article. Review.
-
TAM receptors in cardiovascular disease.Cardiovasc Res. 2019 Jul 1;115(8):1286-1295. doi: 10.1093/cvr/cvz100. Cardiovasc Res. 2019. PMID: 30980657 Free PMC article. Review.
Cited by
-
Contributions of Major Cell Populations to Sjögren's Syndrome.J Clin Med. 2020 Sep 22;9(9):3057. doi: 10.3390/jcm9093057. J Clin Med. 2020. PMID: 32971904 Free PMC article. Review.
-
Advances in cellular and molecular pathways of salivary gland damage in Sjögren's syndrome.Front Immunol. 2024 Jul 10;15:1405126. doi: 10.3389/fimmu.2024.1405126. eCollection 2024. Front Immunol. 2024. PMID: 39050857 Free PMC article. Review.
-
Defective Efferocytosis in a Murine Model of Sjögren's Syndrome Is Mediated by Dysfunctional Mer Tyrosine Kinase Receptor.Int J Mol Sci. 2021 Sep 8;22(18):9711. doi: 10.3390/ijms22189711. Int J Mol Sci. 2021. PMID: 34575873 Free PMC article.
-
Sjögren's Disease and Gastroesophageal Reflux Disease: What Is Their Evidence-Based Link?Medicina (Kaunas). 2024 Nov 18;60(11):1894. doi: 10.3390/medicina60111894. Medicina (Kaunas). 2024. PMID: 39597079 Free PMC article. Review.
-
Research progress on inflammatory mechanism of primary Sjögren syndrome.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Dec 25;50(6):783-794. doi: 10.3724/zdxbyxb-2021-0072. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 35347914 Free PMC article. Review. English.
References
-
- Shiboski C. H., Shiboski S. C., Seror R., et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis & Rheumatology. 2017;69(1):35–45. doi: 10.1002/art.39859. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

