Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing
- PMID: 31214894
- PMCID: PMC6726830
- DOI: 10.1007/s10974-019-09529-7
Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing
Abstract
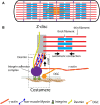
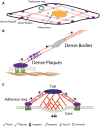
The stiffness of the cardiovascular environment changes during ageing and in disease and contributes to disease incidence and progression. For instance, increased arterial stiffness can lead to atherosclerosis, while stiffening of the heart due to fibrosis can increase the chances of heart failure. Cells can sense the stiffness of the extracellular matrix through integrin adhesions and other mechanosensitive structures and in response to this initiate mechanosignalling pathways that ultimately change the cellular behaviour. Over the past decades, interest in mechanobiology has steadily increased and with this also our understanding of the molecular basis of mechanosensing and transduction. However, much of our knowledge about the mechanisms is derived from studies investigating focal adhesions in non-muscle cells, which are distinct in several regards from the cell-matrix adhesions in cardiomyocytes (costameres) or vascular smooth muscle cells (dense plaques or podosomes). Therefore, we will look here first at the evidence for mechanical sensing in the cardiovascular system, before comparing the different cytoskeletal arrangements and adhesion sites in cardiomyocytes and vascular smooth muscle cells and what is known about mechanical sensing through the various structures.
Keywords: Cardiomyocytes; Costameres; Dense plaques; Mechanosensing; Podosomes; Smooth muscle cells.
Figures
Similar articles
-
Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease.Physiol Rev. 2017 Oct 1;97(4):1555-1617. doi: 10.1152/physrev.00003.2017. Physiol Rev. 2017. PMID: 28954852 Review.
-
Mechanotransduction at the cell-matrix interface.Semin Cell Dev Biol. 2017 Nov;71:75-83. doi: 10.1016/j.semcdb.2017.07.027. Epub 2017 Jul 25. Semin Cell Dev Biol. 2017. PMID: 28754442 Review.
-
Mining the Stiffness-Sensitive Transcriptome in Human Vascular Smooth Muscle Cells Identifies Long Noncoding RNA Stiffness Regulators.Arterioscler Thromb Vasc Biol. 2018 Jan;38(1):164-173. doi: 10.1161/ATVBAHA.117.310237. Epub 2017 Oct 19. Arterioscler Thromb Vasc Biol. 2018. PMID: 29051139 Free PMC article.
-
Podosomes in adhesion, migration, mechanosensing and matrix remodeling.Cytoskeleton (Hoboken). 2013 Oct;70(10):572-89. doi: 10.1002/cm.21119. Epub 2013 Jul 19. Cytoskeleton (Hoboken). 2013. PMID: 23804547 Review.
-
Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-muscle Myosin Contractions.Dev Cell. 2018 Feb 5;44(3):326-336.e3. doi: 10.1016/j.devcel.2017.12.024. Epub 2018 Jan 26. Dev Cell. 2018. PMID: 29396114 Free PMC article.
Cited by
-
Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage.J Muscle Res Cell Motil. 2021 Jun;42(2):367-380. doi: 10.1007/s10974-021-09596-9. Epub 2021 Feb 17. J Muscle Res Cell Motil. 2021. PMID: 33595762 Free PMC article.
-
The Role of Integrin β1D Mislocalization in the Pathophysiology of Calpain 3-Related Limb-Girdle Muscular Dystrophy.Cells. 2025 Mar 17;14(6):446. doi: 10.3390/cells14060446. Cells. 2025. PMID: 40136695 Free PMC article.
-
The intercalated disc: a mechanosensing signalling node in cardiomyopathy.Biophys Rev. 2020 Aug;12(4):931-946. doi: 10.1007/s12551-020-00737-x. Epub 2020 Jul 13. Biophys Rev. 2020. PMID: 32661904 Free PMC article. Review.
-
Pressure and stiffness sensing together regulate vascular smooth muscle cell phenotype switching.Sci Adv. 2022 Apr 15;8(15):eabm3471. doi: 10.1126/sciadv.abm3471. Epub 2022 Apr 15. Sci Adv. 2022. PMID: 35427166 Free PMC article.
-
Profiling the responsiveness of focal adhesions of human cardiomyocytes to extracellular dynamic nano-topography.Bioact Mater. 2021 Aug 28;10:367-377. doi: 10.1016/j.bioactmat.2021.08.028. eCollection 2022 Apr. Bioact Mater. 2021. PMID: 34901553 Free PMC article.
References
-
- Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996;94:3263–3270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

