Development of an oral reference dose for the perfluorinated compound GenX
- PMID: 31215065
- PMCID: PMC6771874
- DOI: 10.1002/jat.3812
Development of an oral reference dose for the perfluorinated compound GenX
Abstract
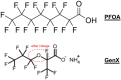
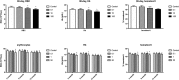
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate, also known as GenX, is a processing aid used in the manufacture of fluoropolymers. GenX is one of several chemistries developed as an alternative to long-chain poly-fluoroalkyl substances, which tend to have long clearance half-lives and are environmentally persistent. Unlike poly-fluoroalkyl substances, GenX has more rapid clearance, but has been detected in US and international water sources. There are currently no federal drinking water standards for GenX in the USA; therefore, we developed a non-cancer oral reference dose (RfD) for GenX based on available repeated dose studies. The review of the available data indicate that GenX is unlikely to be genotoxic. A combination of traditional frequentist benchmark dose models and Bayesian benchmark dose models were used derive relevant points of departure from mammalian toxicity studies. In addition, deterministic and probabilistic RfD values were developed using available tools and regulatory guidance. The two approaches resulted in a narrow range of RfD values for liver lesions observed in a 2-year bioassay in rats (0.01-0.02 mg/kg/day). The probabilistic approach resulted in the lower, i.e., more conservative RfD. The probabilistic RfD of 0.01 mg/kg/day results in a maximum contaminant level goal of 70 ppb. It is anticipated that these values, along with the hazard identification and dose-response modeling described herein, should be informative for risk assessors and regulators interested in setting health-protective drinking water guideline values for GenX.
Keywords: GenX; benchmark dose (BMD) modeling; per- and polyfluoroalkyl substances (PFAS); reference dose (RfD); risk assessment.
© 2019 The Authors Journal of Applied Toxicology Published by John Wiley & Sons Ltd.
Conflict of interest statement
Employment affiliations of the authors are shown above. ToxStrategies is a private consulting firm providing services to private and public organizations on toxicology and risk assessment issues. This work was supported by the Chemours Company FC, LLC. Chemours was given the opportunity to review the draft manuscript. The purpose of this review was for the authors to receive input on the clarity of the science presented but not on the interpretation of research results. The authors’ scientific conclusions and professional judgments were not subject to the funder's control; the contents of this manuscript reflect solely the view of the authors.
Figures




References
-
- Abbott, B. D. , Wolf, C. J. , Schmid, J. E. , Das, K. P. , Zehr, R. D. , Helfant, L. , … Lau, C. (2007). Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor‐alpha. Toxicological Sciences, 98, 571–581. 10.1093/toxsci/kfm110 - DOI - PubMed
-
- ATSDR . (2018). Toxicological profile for rerfluoroalkyls: Draft for public comment. Agency for Toxic Substances and Disease Registry.
-
- Beekman, M. , Zweers P., de Vries, W. , Janssen, P. , & Zeilmaker, M. (2016). Evaluation of substances used in the GenX technology by Chemours, Dordrecht. National Institute for Public Health and the Environment, RIVM Letter Report 2016–0174.
-
- Boverhof, D. R. , Ladics, G. , Luebke, B. , Botham, J. , Corsini, E. , Evans, E. , … Yang, Y. (2014). Approaches and considerations for the assessment of immunotoxicity for environmental chemicals: a workshop summary. Regulatory Toxicology and Pharmacology, 68, 96–107. 10.1016/j.yrtph.2013.11.012 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

