An evaluation of WHO emergency guidelines for Zika virus disease
- PMID: 31215148
- PMCID: PMC6771472
- DOI: 10.1111/jebm.12347
An evaluation of WHO emergency guidelines for Zika virus disease
Abstract
Background: In the face of an unclear causal association between Zika virus in utero exposure and congenital abnormalities and urgent demand for guidance, the World Health Organization (WHO) had to produce timely and trustworthy guidelines during the 2016 Public Health Emergency of International Concern (PHEIC).
Methods: This is a cross-sectional evaluation of WHO emergency guidelines produced during the Zika virus disease PHEIC from 1 February to 18 November 2016. We assessed adherence to WHO publication requirements and the reporting of guideline development processes associated with trustworthiness. In the absence of quality appraisal tools for guidelines developed under compressed timeframes, we applied the Appraisal of Guidelines for Research and Evaluation (AGREE II) tool.
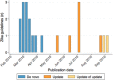
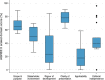
Results: We included 21 guidelines (13 de novo and 8 updates). Six guidelines used a formal evidence review process. Most guidelines involved external experts in the development process and collected declarations of interest. Peer review was reported in six documents. Most emergency guidelines included updating plans. The highest scoring AGREE II domain was clarity of presentation (median score 78%); the lowest scoring domain was applicability (median score 18%).
Conclusion: WHO developed moderate- to high-quality emergency guidelines in the challenging context of a PHEIC. We found improvement opportunities for WHO guideline development teams in the use of evidence to formulate recommendations, the collection of declarations of interest, reporting of conflicts of interest, and the use of existing WHO organizational quality assurance processes.
Keywords: AGREE II; World Health Organization; Zika virus disease; evaluation studies; evidence-based practice; global health; guidelines; methods; quality control.
© 2019 The Authors. Journal of Evidence-Based Medicine published by Chinese Cochrane Center, West China Hospital of Sichuan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
SLN is a member of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group and has published numerous papers related to GRADE. GRADE is the guideline process used by her employer, the World Health Organization, to develop guidelines. MF is currently an employee of Bristol Myers Squibb and owns company stock as part of his remuneration plan; a family member was employed by Bristol Myers Squibb at the time this study was performed. YHAL is currently an employee at Genentech and owns company stock as part of her renumeration; she had no affiliation with Genentech at the time the study was performed. The other authors declare no competing interests.
Figures
Similar articles
-
An evaluation of emergency guidelines issued by the World Health Organization in response to four infectious disease outbreaks.PLoS One. 2018 May 30;13(5):e0198125. doi: 10.1371/journal.pone.0198125. eCollection 2018. PLoS One. 2018. PMID: 29847593 Free PMC article.
-
The rise of Zika infection and microcephaly: what can we learn from a public health emergency?Public Health. 2017 Sep;150:87-92. doi: 10.1016/j.puhe.2017.05.008. Epub 2017 Jun 23. Public Health. 2017. PMID: 28651111 Free PMC article. Review.
-
Meeting Report: WHO consultation on considerations for regulatory expectations of Zika virus vaccines for use during an emergency.Vaccine. 2019 Nov 28;37(50):7443-7450. doi: 10.1016/j.vaccine.2016.10.034. Epub 2016 Dec 20. Vaccine. 2019. PMID: 27916410 Review.
-
Identify-Isolate-Inform: A Tool for Initial Detection and Management of Zika Virus Patients in the Emergency Department.West J Emerg Med. 2016 May;17(3):238-44. doi: 10.5811/westjem.2016.3.30188. Epub 2016 Apr 4. West J Emerg Med. 2016. PMID: 27330653 Free PMC article.
-
A quality evaluation of guidelines on five different viruses causing public health emergencies of international concern.Ann Transl Med. 2020 Apr;8(7):500. doi: 10.21037/atm.2020.03.130. Ann Transl Med. 2020. PMID: 32395544 Free PMC article. Review.
Cited by
-
How consistent are the key recommendations, and what is the quality of guidelines and expert consensus regarding paediatric cow's milk protein allergy?Eur J Pediatr. 2024 Aug;183(8):3543-3556. doi: 10.1007/s00431-024-05622-3. Epub 2024 May 29. Eur J Pediatr. 2024. PMID: 38809454
-
Coronavirus Disease 2019 Related Clinical Studies: A Cross-Sectional Analysis.Front Pharmacol. 2020 Sep 2;11:540187. doi: 10.3389/fphar.2020.540187. eCollection 2020. Front Pharmacol. 2020. PMID: 32982751 Free PMC article.
References
-
- WHO . Zika Virus Classification Table, Data as of 15 February 2018. http://apps.who.int/iris/bitstream/handle/10665/260419/zika-classificati... (accessed 10 October 2018).
-
- WHO . Zika virus , Microcephaly and Guillain‐Barre Syndrome situation report. Geneva: World Health Organization; 2017. (http://apps.who.int/iris/bitstream/10665/254714/1/zikasitrep10Mar17-eng.... (accessed 10 October 2018).
-
- WHO . Zika Virus: Key facts 20 July 2018. https://www.who.int/en/news-room/fact-sheets/detail/zika-virus (accessed 27 December 2018).
-
- Pan American Organiztion and the World Health Organization . Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015 ‐ 2018. Cumulative cases. https://www.paho.org/hq/index.php?option=com_docman&view=download&catego... (accessed 27 December 2018).
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016; 374:1552–1563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

