A shared protocol for porcine surfactant use in pediatric acute respiratory distress syndrome: a feasibility study
- PMID: 31215483
- PMCID: PMC6580470
- DOI: 10.1186/s12887-019-1579-3
A shared protocol for porcine surfactant use in pediatric acute respiratory distress syndrome: a feasibility study
Abstract
Background: Pediatric ARDS still represents a difficult challenge in Pediatric Intensive Care Units (PICU). Among different treatments proposed, exogenous surfactant showed conflicting results. Aim of this multicenter retrospective observational study was to evaluate whether poractant alfa use in pediatric ARDS might improve gas exchange in children less than 2 years old, according to a shared protocol.
Methods: The study was carried out in fourteen Italian PICUs after dissemination of a standardized protocol for surfactant administration within the Italian PICU network. The protocol provides the administration of surfactant (50 mg/kg) divided in two doses: the first dose is used as a bronchoalveolar lavage while the second as supplementation. Blood gas exchange variations before and after surfactant use were recorded.
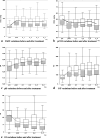
Results: Sixty-nine children, age 0-24 months, affected by Acute Respiratory Distress Syndrome treated with exogenous porcine surfactant were enrolled. Data collection consisted of patient demographics, respiratory variables and arterial blood gas analysis. The most frequent reasons for PICU admission were acute respiratory failure, mainly bronchiolitis and pneumonia, and septic shock. Fifty-four children (78.3%) had severe ARDS (define by oxygen arterial pressure and inspired oxygen fraction ratio (P/F) < 100), 15 (21.7%) had moderate ARDS (100 < P/F < 200). PO2, P/F, Oxygenation Index (OI) and pH showed a significant improvement after surfactant use with respect to baseline (p < 0.001 at each included time-point for each parameter). No significant difference in blood gas variations were observed among four different subgroups of diseases (bronchiolitis, pneumonia, septic shock and others). Overall, 11 children died (15.9%) and among these, 10 (90.9%) had complex chronic conditions. Two children (18.2%) died while being treated with Extracorporeal Membrane Oxygenation (ECMO). Mortality for severe pARDS was 20.4%.
Conclusion: The use of porcine Surfactant improves oxygenation, P/F ratio, OI and pH in a population of children with moderate or severe pARDS caused by multiple diseases. A shared protocol seems to be a good option to obtain the same criteria of enrollment among different PICUs and define a unique way of use and administration of the drug for future studies.
Keywords: Infants; Pediatric intensive care unit; Poractant; Surfactant; pARDS.
Conflict of interest statement
Dr. Santuz received funding from Chiesi Farmaceutici S.p.A. The remaining authors declare that they have no competing interests.
Figures

Similar articles
-
Surfactant improves oxygenation in infants and children with pneumonia and acute respiratory distress syndrome.Acta Paediatr. 2002;91(11):1174-8. doi: 10.1080/080352502320777397. Acta Paediatr. 2002. PMID: 12463314
-
Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study.Yonsei Med J. 2015 Mar;56(2):433-9. doi: 10.3349/ymj.2015.56.2.433. Yonsei Med J. 2015. PMID: 25683992 Free PMC article.
-
Intratracheal atomized surfactant provides similar outcomes as bolus surfactant in preterm lambs with respiratory distress syndrome.Pediatr Res. 2016 Jul;80(1):92-100. doi: 10.1038/pr.2016.39. Epub 2016 Mar 8. Pediatr Res. 2016. PMID: 26954481
-
Surfactant from neonatal to pediatric ICU: bench and bedside evidence.Minerva Anestesiol. 2014 Dec;80(12):1345-56. Epub 2014 Feb 7. Minerva Anestesiol. 2014. PMID: 24504167 Review.
-
The role of surfactant treatment in preterm infants and term newborns with acute respiratory distress syndrome.J Perinatol. 2009 May;29 Suppl 2:S18-22. doi: 10.1038/jp.2009.30. J Perinatol. 2009. PMID: 19399004 Review.
Cited by
-
Effect of comprehensive nursing intervention in children with respiratory failure.Am J Transl Res. 2022 Oct 15;14(10):7217-7225. eCollection 2022. Am J Transl Res. 2022. PMID: 36398203 Free PMC article.
-
Bronchoscopic lung lavage and exogenous surfactant successfully reverse respiratory failure after severe chlorine exposure: A pediatric case report.Clin Case Rep. 2025 Jan 14;13(1):e9302. doi: 10.1002/ccr3.9302. eCollection 2025 Jan. Clin Case Rep. 2025. PMID: 39810993 Free PMC article.
-
Correlation Between the Ratio of Oxygen Saturation to Fraction of Inspired Oxygen and the Ratio of Partial Pressure of Oxygen to Fraction of Inspired Oxygen in Detection and Risk Stratification of Pediatric Acute Respiratory Distress Syndrome.Cureus. 2021 Sep 28;13(9):e18353. doi: 10.7759/cureus.18353. eCollection 2021 Sep. Cureus. 2021. PMID: 34725605 Free PMC article.
-
Efficacy and safety of exogenous surfactant therapy in patients under 12 months of age invasively ventilated for severe bronchiolitis (SURFABRON): protocol for a multicentre, randomised, double-blind, controlled, non-profit trial.BMJ Open. 2020 Oct 19;10(10):e038780. doi: 10.1136/bmjopen-2020-038780. BMJ Open. 2020. PMID: 33077567 Free PMC article.
-
Effect of pulmonary surfactant on the prevention of neonatal respiratory distress syndrome in premature infants.Am J Transl Res. 2021 Apr 15;13(4):3642-3649. eCollection 2021. Am J Transl Res. 2021. PMID: 34017546 Free PMC article.
References
-
- Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric acute lung injury consensus conference group: pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S23–S40. doi: 10.1097/PCC.0000000000000432. - DOI - PubMed
-
- Notter RH. Lung surfactant dysfunction and disease of lung surfactant deficiency or dysfunction. In: Dekker M, editor. Lung surfactants: basic science and clinical applications. New York. 2000. pp. 207–247.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

