Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells
- PMID: 31215502
- PMCID: PMC6582495
- DOI: 10.1186/s13046-019-1264-2
Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells
Abstract
Background: Glioblastoma (GBM) is an aggressive brain tumor with universal recurrence and poor prognosis. The recurrence is largely driven by chemoradiation resistant cancer stem cells (CSCs). Epidermal growth factor receptor (EGFR) and its mutant EGFRvIII are amplified in ~ 60% and ~ 30% of GBM patients, respectively; however, therapies targeting EGFR have failed to improve disease outcome. EGFRvIII-mediated cross-activation of tyrosine kinase receptor, cMET, regulates GBM CSC maintenance and promote tumor recurrence. Here, we evaluated the efficacy of pan-EGFR inhibitor afatinib and Temozolomide (TMZ) combination on GBM in vitro and in vivo.
Methods: We analyzed the effect of afatinib and temozolomide (TMZ) combination on GBM cells U87MG and U251 engineered to express wild type (WT) EGFR, EGFRvIII or EGFRvIII dead kinase, CSCs isolated from U87 and U87EGFRvIII in vitro. The therapeutic utility of the drug combination was investigated on tumor growth and progression using intracranially injected U87EGFRvIII GBM xenografts.
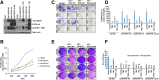
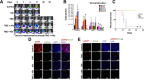
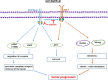
Results: Afatinib and TMZ combination synergistically inhibited the proliferation, clonogenic survival, motility, invasion and induced senescence of GBM cells compared to monotherapy. Mechanistically, afatinib decreased U87EGFRvIII GBM cell proliferation and motility/invasion by inhibiting EGFRvIII/AKT, EGFRvIII/JAK2/STAT3, and focal adhesion kinase (FAK) signaling pathways respectively. Interestingly, afatinib specifically inhibited EGFRvIII-cMET crosstalk in CSCs, resulting in decreased expression of Nanog and Oct3/4, and in combination with TMZ significantly decreased their self-renewal property in vitro. More interestingly, afatinib and TMZ combination significantly decreased the xenograft growth and progression compared to single drug alone.
Conclusion: Our study demonstrated significant inhibition of GBM tumorigenicity, CSC maintenance in vitro, and delayed tumor growth and progression in vivo by combination of afatinib and TMZ. Our results warrant evaluation of this drug combination in EGFR and EGFRvIII amplified GBM patients.
Keywords: Afatinib; Cancer stem cells; Epidermal growth factor receptor; Glioblastoma; Temozolomide.
Conflict of interest statement
SKB is one of the co-founder of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflict of interest.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neuro-Oncol. 2012;107(2):359–364. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

